Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
108 Chapter 2 • Chemical <strong>Stability</strong> <strong>of</strong> Drug Substances<br />
shaped crystals. 409 Solid-state hydrolysis <strong>of</strong> carbamazepine from needle-shaped crystals<br />
with a higher Crystalline order is faster than that <strong>of</strong> beam-shaped <strong>and</strong> prismatic forms. 410<br />
Reactivity <strong>of</strong> carbamazepine to light also depends on the crystalline form <strong>of</strong> the drug. 411<br />
Differences in reactivity among different crystalline forms have also been reported for<br />
photodegradation <strong>of</strong> furosemide. 412<br />
The stability <strong>of</strong> drugs in their amorphous form is generally lower than that <strong>of</strong> drugs in<br />
their crystalline form, because <strong>of</strong> the higher free-energy level <strong>of</strong> the amorphous state. The<br />
relationship between degradation rate <strong>and</strong> crystallinity determined from heats <strong>of</strong> dissolution<br />
for ß-lactam antibiotics such as sodium cefazolin indicates that a drug with low crystallinity<br />
tends to have decreased chemical stability. 413<br />
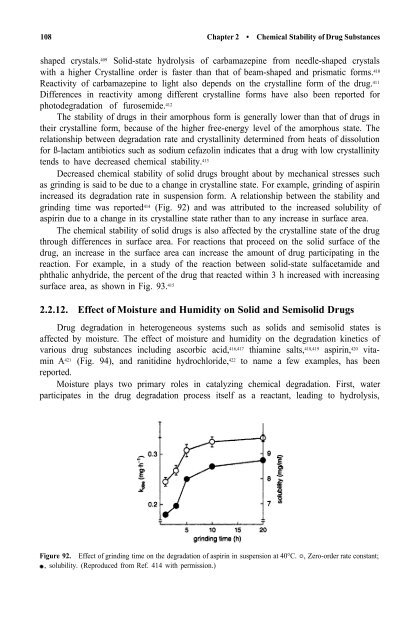
Decreased chemical stability <strong>of</strong> solid drugs brought about by mechanical stresses such<br />
as grinding is said to be due to a change in crystalline state. For example, grinding <strong>of</strong> aspirin<br />
increased its degradation rate in suspension form. A relationship between the stability <strong>and</strong><br />
grinding time was reported 414 (Fig. 92) <strong>and</strong> was attributed to the increased solubility <strong>of</strong><br />
aspirin due to a change in its crystalline state rather than to any increase in surface area.<br />
The chemical stability <strong>of</strong> solid drugs is also affected by the crystalline state <strong>of</strong> the drug<br />
through differences in surface area. For reactions that proceed on the solid surface <strong>of</strong> the<br />
drug, an increase in the surface area can increase the amount <strong>of</strong> drug participating in the<br />
reaction. For example, in a study <strong>of</strong> the reaction between solid-state sulfacetamide <strong>and</strong><br />
phthalic anhydride, the percent <strong>of</strong> the drug that reacted within 3 h increased with increasing<br />
surface area, as shown in Fig. 93. 415<br />
2.2.12. Effect <strong>of</strong> Moisture <strong>and</strong> Humidity on Solid <strong>and</strong> Semisolid <strong>Drugs</strong><br />
Drug degradation in heterogeneous systems such as solids <strong>and</strong> semisolid states is<br />
affected by moisture. The effect <strong>of</strong> moisture <strong>and</strong> humidity on the degradation kinetics <strong>of</strong><br />
various drug substances including ascorbic acid, 416,417 thiamine salts, 418,419 aspirin, 420 vitamin<br />
A 421 (Fig. 94), <strong>and</strong> ranitidine hydrochloride, 422 to name a few examples, has been<br />
reported.<br />
Moisture plays two primary roles in catalyzing chemical degradation. First, water<br />
participates in the drug degradation process itself as a reactant, leading to hydrolysis,<br />
Figure 92. Effect <strong>of</strong> grinding time on the degradation <strong>of</strong> aspirin in suspension at 40°C. , Zero-order rate constant;<br />
, solubility. (Reproduced from Ref. 414 with permission.)