Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
120 Chapter 2 • Chemical <strong>Stability</strong> <strong>of</strong> Drug Substances<br />
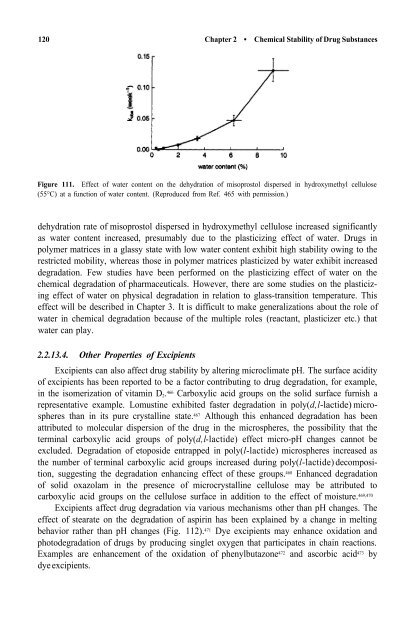
Figure 111. Effect <strong>of</strong> water content on the dehydration <strong>of</strong> misoprostol dispersed in hydroxymethyl cellulose<br />
(55°C) at a function <strong>of</strong> water content. (Reproduced from Ref. 465 with permission.)<br />
dehydration rate <strong>of</strong> misoprostol dispersed in hydroxymethyl cellulose increased significantly<br />
as water content increased, presumably due to the plasticizing effect <strong>of</strong> water. <strong>Drugs</strong> in<br />
polymer matrices in a glassy state with low water content exhibit high stability owing to the<br />
restricted mobility, whereas those in polymer matrices plasticized by water exhibit increased<br />
degradation. Few studies have been performed on the plasticizing effect <strong>of</strong> water on the<br />
chemical degradation <strong>of</strong> pharmaceuticals. However, there are some studies on the plasticizing<br />
effect <strong>of</strong> water on physical degradation in relation to glass-transition temperature. This<br />
effect will be described in Chapter 3. It is difficult to make generalizations about the role <strong>of</strong><br />
water in chemical degradation because <strong>of</strong> the multiple roles (reactant, plasticizer etc.) that<br />
water can play.<br />
2.2.13.4. Other Properties <strong>of</strong> Excipients<br />
Excipients can also affect drug stability by altering microclimate pH. The surface acidity<br />
<strong>of</strong> excipients has been reported to be a factor contributing to drug degradation, for example,<br />
in the isomerization <strong>of</strong> vitamin D 2 . 466 Carboxylic acid groups on the solid surface furnish a<br />
representative example. Lomustine exhibited faster degradation in poly( d,l-lactide) microspheres<br />
than in its pure crystalline state. 467 Although this enhanced degradation has been<br />
attributed to molecular dispersion <strong>of</strong> the drug in the microspheres, the possibility that the<br />
terminal carboxylic acid groups <strong>of</strong> poly( d,l-lactide) effect micro-pH changes cannot be<br />
excluded. Degradation <strong>of</strong> etoposide entrapped in poly( l-lactide) microspheres increased as<br />
the number <strong>of</strong> terminal carboxylic acid groups increased during poly( l-lactide) decomposition,<br />
suggesting the degradation enhancing effect <strong>of</strong> these groups. 468 Enhanced degradation<br />
<strong>of</strong> solid oxazolam in the presence <strong>of</strong> microcrystalline cellulose may be attributed to<br />
carboxylic acid groups on the cellulose surface in addition to the effect <strong>of</strong> moisture. 469,470<br />
Excipients affect drug degradation via various mechanisms other than pH changes. The<br />
effect <strong>of</strong> stearate on the degradation <strong>of</strong> aspirin has been explained by a change in melting<br />
behavior rather than pH changes (Fig. 112). 471 Dye excipients may enhance oxidation <strong>and</strong><br />
photodegradation <strong>of</strong> drugs by producing singlet oxygen that participates in chain reactions.<br />
Examples are enhancement <strong>of</strong> the oxidation <strong>of</strong> phenylbutazone 472 <strong>and</strong> ascorbic acid 473 by<br />
dye excipients.