Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4.1. • Preformulation <strong>and</strong> Formulation <strong>Stability</strong> Studies 153<br />
Thermal analysis is <strong>of</strong>ten capable <strong>of</strong> easily detecting drug-excipient interactions. For<br />
example, accelerated degradation <strong>of</strong> aspirin caused by physical mixture with silica <strong>and</strong><br />
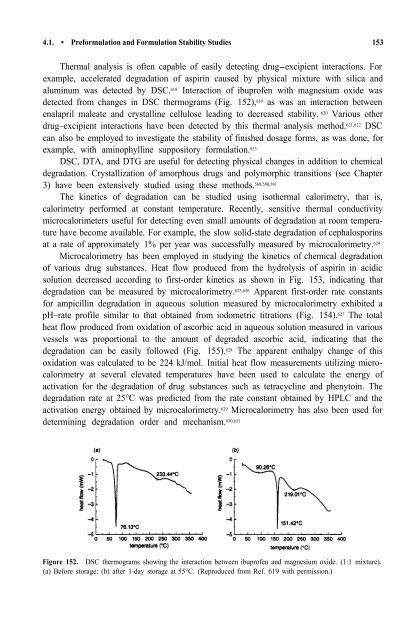
aluminum was detected by DSC. 618 Interaction <strong>of</strong> ibupr<strong>of</strong>en with magnesium oxide was<br />
detected from changes in DSC thermograms (Fig. 152), 619 as was an interaction between<br />
enalapril maleate <strong>and</strong> crystalline cellulose leading to decreased stability. 620 Various other<br />
drug–excipient interactions have been detected by this thermal analysis method. 621,622 DSC<br />
can also be employed to investigate the stability <strong>of</strong> finished dosage forms, as was done, for<br />
example, with aminophylline suppository formulation. 623<br />
DSC, DTA, <strong>and</strong> DTG are useful for detecting physical changes in addition to chemical<br />
degradation. Crystallization <strong>of</strong> amorphous drugs <strong>and</strong> polymorphic transitions (see Chapter<br />
3) have been extensively studied using these methods. 568,580,581<br />
The kinetics <strong>of</strong> degradation can be studied using isothermal calorimetry, that is,<br />
calorimetry performed at constant temperature. Recently, sensitive thermal conductivity<br />
microcalorimeters useful for detecting even small amounts <strong>of</strong> degradation at room temperature<br />
have become available. For example, the slow solid-state degradation <strong>of</strong> cephalosporins<br />
at a rate <strong>of</strong> approximately 1% per year was successfully measured by microcalorimetry. 624<br />
Microcalorimetry has been employed in studying the kinetics <strong>of</strong> chemical degradation<br />
<strong>of</strong> various drug substances. Heat flow produced from the hydrolysis <strong>of</strong> aspirin in acidic<br />
solution decreased according to first-order kinetics as shown in Fig. 153, indicating that<br />
degradation can be measured by microcalorimetry. 625,626 Apparent first-order rate constants<br />
for ampicillin degradation in aqueous solution measured by microcalorimetry exhibited a<br />
pH–rate pr<strong>of</strong>ile similar to that obtained from iodometric titrations (Fig. 154). 627 The total<br />
heat flow produced from oxidation <strong>of</strong> ascorbic acid in aqueous solution measured in various<br />
vessels was proportional to the amount <strong>of</strong> degraded ascorbic acid, indicating that the<br />
degradation can be easily followed (Fig. 155). 628 The apparent enthalpy change <strong>of</strong> this<br />
oxidation was calculated to be 224 kJ/mol. Initial heat flow measurements utilizing microcalorimetry<br />
at several elevated temperatures have been used to calculate the energy <strong>of</strong><br />
activation for the degradation <strong>of</strong> drug substances such as tetracycline <strong>and</strong> phenytoin. The<br />
degradation rate at 25°C was predicted from the rate constant obtained by HPLC <strong>and</strong> the<br />
activation energy obtained by microcalorimetry. 629 Microcalorimetry has also been used for<br />
determining degradation order <strong>and</strong> mechanism. 630,631<br />
Figure 152. DSC thermograms showing the interaction between ibupr<strong>of</strong>en <strong>and</strong> magnesium oxide. (1:1 mixture).<br />
(a) Before storage; (b) after 1-day storage at 55°C. (Reproduced from Ref. 619 with permission.)