Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.3. • Stabilization <strong>of</strong> Drug Substances against Chemical Degradation 127<br />
The term [D–L] represents the concentration <strong>of</strong> the complex, D–L, [D] ƒ is the concentration<br />
<strong>of</strong> free or uncomplexed drug, <strong>and</strong> [L] ƒ is the concentration <strong>of</strong> free lig<strong>and</strong>. In Scheme 76, k ƒ<br />
represents the rate constant for the degradation <strong>of</strong> the drug in the absence <strong>of</strong> complexation,<br />
<strong>and</strong> k, is the rate constant for the degradation <strong>of</strong> the drug in its complexed form. As can be<br />
seen, the drug will be stabilized by the presence <strong>of</strong> L if k c < k ƒ . The degree <strong>of</strong> stabilization<br />
will also depend on the relative amounts <strong>of</strong> free <strong>and</strong> complexed drug, which in turn depends<br />
on the concentrations <strong>of</strong> D <strong>and</strong> L <strong>and</strong> the magnitude <strong>of</strong> K. Conversely, if k c > k ƒ , complex<br />
formation will result in acceleration <strong>of</strong> the degradation. Differing lig<strong>and</strong>s (L) in a series can<br />
affect the degradation rate in two ways: first, by affecting the degree <strong>of</strong> complexation, as<br />
measured by K, <strong>and</strong>, second, by affecting k c .<br />
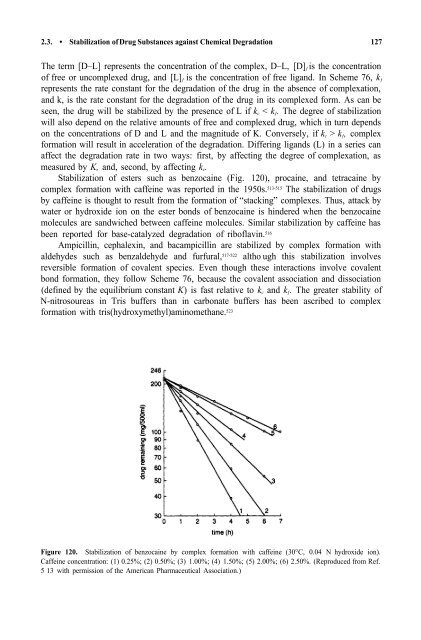
Stabilization <strong>of</strong> esters such as benzocaine (Fig. 120), procaine, <strong>and</strong> tetracaine by<br />
complex formation with caffeine was reported in the 1950s. 513-515 The stabilization <strong>of</strong> drugs<br />
by caffeine is thought to result from the formation <strong>of</strong> “stacking” complexes. Thus, attack by<br />
water or hydroxide ion on the ester bonds <strong>of</strong> benzocaine is hindered when the benzocaine<br />
molecules are s<strong>and</strong>wiched between caffeine molecules. Similar stabilization by caffeine has<br />
been reported for base-catalyzed degradation <strong>of</strong> rib<strong>of</strong>lavin. 516<br />
Ampicillin, cephalexin, <strong>and</strong> bacampicillin are stabilized by complex formation with<br />
aldehydes such as benzaldehyde <strong>and</strong> furfural, 517-522 altho ugh this stabilization involves<br />
reversible formation <strong>of</strong> covalent species. Even though these interactions involve covalent<br />
bond formation, they follow Scheme 76, because the covalent association <strong>and</strong> dissociation<br />
(defined by the equilibrium constant K) is fast relative to k c <strong>and</strong> k ƒ . The greater stability <strong>of</strong><br />
N-nitrosoureas in Tris buffers than in carbonate buffers has been ascribed to complex<br />
formation with tris(hydroxymethyl)aminomethane. 523<br />
Figure 120. Stabilization <strong>of</strong> benzocaine by complex formation with caffeine (30°C, 0.04 N hydroxide ion).<br />
Caffeine concentration: (1) 0.25%; (2) 0.50%; (3) 1.00%; (4) 1.50%; (5) 2.00%; (6) 2.50%. (Reproduced from Ref.<br />
5 13 with permission <strong>of</strong> the American Pharmaceutical Association.)