Problem Set 3
Problem Set 3
Problem Set 3
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
CHEM 153 - Advanced Inorganic Chemistry<br />
PROBLEM SET 3 (due 29 January, 2010)<br />
1. Consider the following oxo complexes:<br />
a. [L 5 VO] 2+ V IV d 1<br />
b. [L 5 CrO] 3+ Cr V d 1<br />
c. [L 5 CrO] 2+ Cr IV d 2<br />
d. [L 5 MnO] 3+ Mn V d 2<br />
e. [L 5 MnO] 2+ Mn IV d 3<br />
f. [L 5 FeO] 2+ Fe IV d 4<br />
Part 1:<br />
Construct an MO diagram for C 4v [L 5 MO] n+ (L is an uncharged ligand, for<br />
example, H 2 O or NH 3 ) using the following orbitals: five metal 3d orbitals, one set<br />
of five ligand F orbitals, and the oxo 2pF + two 2pB orbitals.<br />
Part 2:<br />
Using your MO diagram, for each of the above examples, fill in the d electrons<br />
and predict the ground-state electronic configuration. With this prediction,<br />
calculate the metal-oxo bond order. Based on your results, do you think that a<br />
complex of the form [L 5 CoO] n+ could exist Why or why not<br />
Part 3:<br />
a. Larry Que and coworkers reported the synthesis and isolation of a nonheme<br />
ferryl complex (Science 2003, 299, 1037-1039). What are the Fe-O bond<br />
properties (bond length and bond order) Are they consistent with your<br />
predictions for 6-coordinate Fe IV How do the reported Mössbauer spectra<br />
support these findings<br />
b. The electronic structures and spectroscopic properties of Que’s nonheme<br />
ferryl complex have been investigated in Ed Solomon’s laboratory (J. Am.<br />
Chem. Soc. 2004, 126, 5378-7379). An absorption system in the 10,000 cm !1<br />
region, called band I by Solomon, is assigned to the (xy)6(x 2 !y 2 ) transition in<br />
the ferryl spectrum. What is the electronic configuration of the excited state<br />
formed by this transition What is the value of S (the spin quantum number)<br />
for this excited state The energies of the transitions associated with bands I<br />
and II are at much lower energies than the corresponding transitions in d 1<br />
metal-oxo complexes such as [L 5 VO] 2+ and [L 5 CrO] 3+ . How does Solomon<br />
account for these differences<br />
-1-
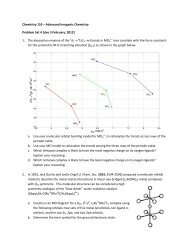
2. In 1993, Wilkinson and Hursthouse reported<br />
(Polyhedron, 1993, 12, 2009-2012) the preparation<br />
and X-ray crystal structure of Ir(O)(mes) 3 (mes =<br />
mesityl, 2,4,6-trimethylphenyl), a complex that lies<br />
beyond the oxo wall. The drawing to the right was<br />
extracted from this paper.<br />
Part 1:<br />
Construct an MO diagram for C 3v Ir(O)(mes) 3 using the following orbitals: five<br />
metal 5d orbitals, one set of three ligand F orbitals, and the oxo 2pF + two 2pB<br />
orbitals.<br />
Part 2:<br />
Using your MO diagram, fill in the d electrons and predict the ground-state<br />
electronic configuration. With this prediction, is the metal-oxo bond order shown<br />
above correct Why or why not Do you think that a complex of the form<br />
[Ir(O)(mes) 3 ] 2! could exist Why or why not<br />
-2-
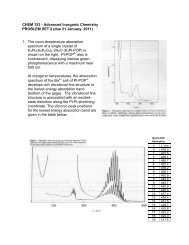
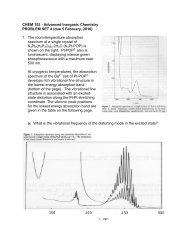
3. The 4K single crystal polarized<br />
electronic absorption spectrum of the<br />
tetragonal molybdenyl ion in<br />
(Ph 4 As)[MoOCl 4 ] is shown to the right.<br />
Part 1:<br />
a. Draw the molecular structure of<br />
the [MoOCl 4 ] ! ion, determine the<br />
oxidation state of the Mo center<br />
and its d-electron count.<br />
b. Construct an LF diagram for<br />
[MoOCl 4 ] ! using the following<br />
orbitals: five Mo 4d orbitals, four<br />
Cl ! F orbitals, eight Cl ! B orbitals<br />
and the oxo F + 2B orbitals.<br />
Give the term symbol for the<br />
ground electronic state.<br />
c. On the basis of your LF diagram, assign the absorption bands with maxima at<br />
~640 and ~430 nm to specific electronic transitions (give the electronic<br />
configurations and state designations for both excited states).<br />
Part 2:<br />
The [MoOCl 4 ] ! ion has 12 normal modes of vibration with the following<br />
symmetries and frequencies (< refers to a stretching mode; B and * refer to<br />
deformation or bending modes):<br />
vibration cm !1<br />
a 1 :
a. On the basis of your assignment of the lower energy electronic absorption<br />
band, in which vibrational mode(s) would you expect to see fine structure<br />
Can you explain the fine structure that appears in the lower energy band<br />
b. An enlarged view of the higher energy band appears at the bottom of the<br />
page. Examine the vibrational fine structure in this band and assign it to a<br />
ground-state vibrational mode. Discuss whether a distortion in this mode is<br />
consistent with the assignment of the electronic transition.<br />
c. Perform a Franck-Condon analysis of the vibrational fine structure of the<br />
higher energy absorption band. What S HR -value gives the best fit to the<br />
observed spectrum If the force constant for the distorting mode is 1.91<br />
mdyne/Å, give the magnitude of the distortion in the normal mode ()Q). Use<br />
group theory to define the normal mode in terms of bond stretching<br />
coordinates to estimate the magnitude of the distortion in the individual<br />
bonds.<br />
-4-