in vivo
in vivo
in vivo
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
How eat<strong>in</strong>g cell ‘corpses’ reduces<br />
<strong>in</strong>flammation<br />
Specialized immune cells orchestrate proper elim<strong>in</strong>ation of dead cells to<br />
prevent <strong>in</strong>flammation<br />
Masato Tanaka<br />
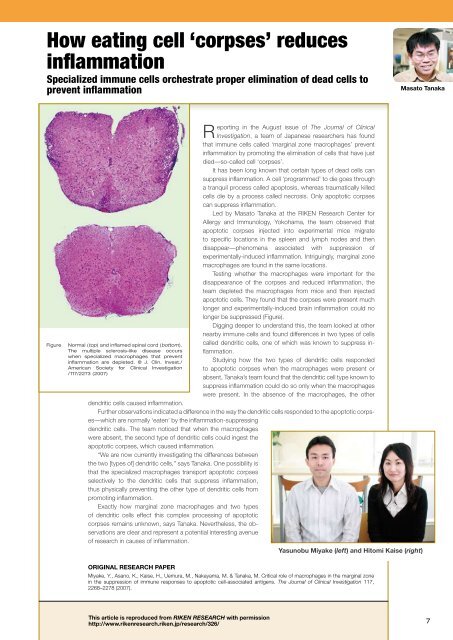
Figure<br />
Normal (top) and <strong>in</strong>flamed sp<strong>in</strong>al cord (bottom).<br />
The multiple sclerosis-like disease occurs<br />
when specialized macrophages that prevent<br />
<strong>in</strong>flammation are depleted. © J. Cl<strong>in</strong>. Invest./<br />
American Society for Cl<strong>in</strong>ical Investigation<br />
/117/2273 (2007)<br />
Report<strong>in</strong>g <strong>in</strong> the August issue of The Journal of Cl<strong>in</strong>ical<br />
Investigation, a team of Japanese researchers has found<br />
that immune cells called ‘marg<strong>in</strong>al zone macrophages’ prevent<br />
<strong>in</strong>flammation by promot<strong>in</strong>g the elim<strong>in</strong>ation of cells that have just<br />
died—so-called cell ‘corpses’.<br />
It has been long known that certa<strong>in</strong> types of dead cells can<br />
suppress <strong>in</strong>flammation. A cell ‘programmed’ to die goes through<br />
a tranquil process called apoptosis, whereas traumatically killed<br />
cells die by a process called necrosis. Only apoptotic corpses<br />
can suppress <strong>in</strong>flammation.<br />
Led by Masato Tanaka at the RIKEN Research Center for<br />
Allergy and Immunology, Yokohama, the team observed that<br />
apoptotic corpses <strong>in</strong>jected <strong>in</strong>to experimental mice migrate<br />
to specific locations <strong>in</strong> the spleen and lymph nodes and then<br />
disappear—phenomena associated with suppression of<br />
experimentally-<strong>in</strong>duced <strong>in</strong>flammation. Intrigu<strong>in</strong>gly, marg<strong>in</strong>al zone<br />
macrophages are found <strong>in</strong> the same locations.<br />
Test<strong>in</strong>g whether the macrophages were important for the<br />
disappearance of the corpses and reduced <strong>in</strong>flammation, the<br />
team depleted the macrophages from mice and then <strong>in</strong>jected<br />
apoptotic cells. They found that the corpses were present much<br />
longer and experimentally-<strong>in</strong>duced bra<strong>in</strong> <strong>in</strong>flammation could no<br />
longer be suppressed (Figure).<br />
Digg<strong>in</strong>g deeper to understand this, the team looked at other<br />
nearby immune cells and found differences <strong>in</strong> two types of cells<br />
called dendritic cells, one of which was known to suppress <strong>in</strong>flammation.<br />
Study<strong>in</strong>g how the two types of dendritic cells responded<br />
to apoptotic corpses when the macrophages were present or<br />
absent, Tanaka’s team found that the dendritic cell type known to<br />
suppress <strong>in</strong>flammation could do so only when the macrophages<br />
were present. In the absence of the macrophages, the other<br />
dendritic cells caused <strong>in</strong>flammation.<br />
Further observations <strong>in</strong>dicated a difference <strong>in</strong> the way the dendritic cells responded to the apoptotic corpses––which<br />
are normally ‘eaten’ by the <strong>in</strong>flammation-suppress<strong>in</strong>g<br />
dendritic cells. The team noticed that when the macrophages<br />
were absent, the second type of dendritic cells could <strong>in</strong>gest the<br />
apoptotic corpses, which caused <strong>in</strong>flammation.<br />
“We are now currently <strong>in</strong>vestigat<strong>in</strong>g the differences between<br />
the two [types of] dendritic cells,” says Tanaka. One possibility is<br />
that the specialized macrophages transport apoptotic corpses<br />
selectively to the dendritic cells that suppress <strong>in</strong>flammation,<br />
thus physically prevent<strong>in</strong>g the other type of dendritic cells from<br />
promot<strong>in</strong>g <strong>in</strong>flammation.<br />
Exactly how marg<strong>in</strong>al zone macrophages and two types<br />
of dendritic cells effect this complex process<strong>in</strong>g of apoptotic<br />
corpses rema<strong>in</strong>s unknown, says Tanaka. Nevertheless, the observations<br />
are clear and represent a potential <strong>in</strong>terest<strong>in</strong>g avenue<br />
of research <strong>in</strong> causes of <strong>in</strong>flammation.<br />
Yasunobu Miyake (left) and Hitomi Kaise (right)<br />
ORIGINAL RESEARCH PAPER<br />
Miyake, Y., Asano, K., Kaise, H., Uemura, M., Nakayama, M. & Tanaka, M. Critical role of macrophages <strong>in</strong> the marg<strong>in</strong>al zone<br />
<strong>in</strong> the suppression of immune responses to apoptotic cell-associated antigens. The Journal of Cl<strong>in</strong>ical Investigation 117,<br />
2268–2278 (2007).<br />
This article is reproduced from RIKEN RESEARCH with permission<br />
http://www.rikenresearch.riken.jp/research/326/<br />
7