in vivo
in vivo
in vivo
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Role of TLR-MyD88 signals for B cell<br />
recruitment, activation and class switch<br />
recomb<strong>in</strong>ation <strong>in</strong> Peyer’s patch germ<strong>in</strong>al<br />
centers.<br />
Germ<strong>in</strong>al centers (GCs) are recognized as<br />
the ma<strong>in</strong> sites for generation of IgA <strong>in</strong> the Peyer’s<br />
patch (PP), yet the mechanisms that control B<br />
cell recruitment, activation, and IgA production<br />
<strong>in</strong> GCs are not completely understood.<br />
We found that bacterial stimulation through<br />
TLR-MyD88 regulates follicular maturation<br />
and GC reaction <strong>in</strong> the gut immune system.<br />
Unexpectedly, we found that TLR-stimulation of<br />
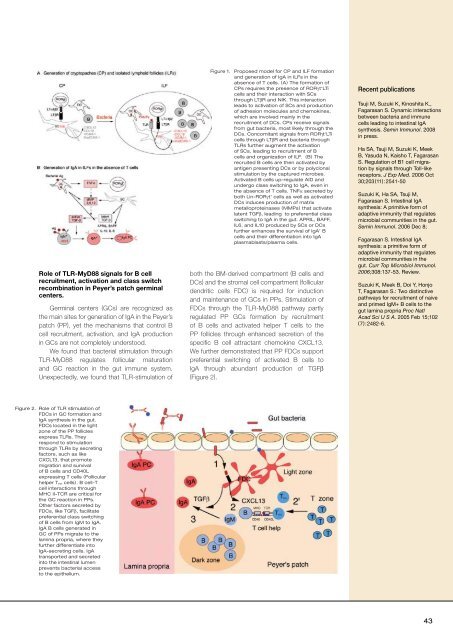
Figure 1. Proposed model for CP and ILF formation<br />
and generation of IgA <strong>in</strong> ILFs <strong>in</strong> the<br />
absence of T cells. (A) The formation of<br />
CPs requires the presence of RORγt + LTi<br />
cells and their <strong>in</strong>teraction with SCs<br />
through LTβR and NIK. This <strong>in</strong>teraction<br />
leads to activation of SCs and production<br />
of adhesion molecules and chemok<strong>in</strong>es,<br />
which are <strong>in</strong>volved ma<strong>in</strong>ly <strong>in</strong> the<br />
recruitment of DCs. CPs receive signals<br />
from gut bacteria, most likely through the<br />
DCs. Concomitant signals from RORγt + LTi<br />
cells through LTβR and bacteria through<br />
TLRs further augment the activation<br />
of SCs, lead<strong>in</strong>g to recruitment of B<br />
cells and organization of ILF. (B) The<br />
recruited B cells are then activated by<br />
antigen present<strong>in</strong>g DCs or by polyclonal<br />
stimulation by the captured microbes.<br />
Activated B cells up-regulate AID and<br />
undergo class switch<strong>in</strong>g to IgA, even <strong>in</strong><br />
the absence of T cells. TNFα secreted by<br />
both L<strong>in</strong>-RORγt + cells as well as activated<br />
DCs <strong>in</strong>duces production of matrix<br />
metalloprote<strong>in</strong>ases (MMPs) that activate<br />
latent TGFβ, lead<strong>in</strong>g to preferential class<br />
switch<strong>in</strong>g to IgA <strong>in</strong> the gut. APRIL, BAFF,<br />
IL6, and IL10 produced by SCs or DCs<br />
further enhances the survival of IgA + B<br />
cells and their differentiation <strong>in</strong>to IgA<br />
plasmablasts/plasma cells.<br />
both the BM-derived compartment (B cells and<br />
DCs) and the stromal cell compartment (follicular<br />
dendritic cells FDC) is required for <strong>in</strong>duction<br />
and ma<strong>in</strong>tenance of GCs <strong>in</strong> PPs. Stimulation of<br />
FDCs through the TLR-MyD88 pathway partly<br />
regulated PP GCs formation by recruitment<br />
of B cells and activated helper T cells to the<br />
PP follicles through enhanced secretion of the<br />
specific B cell attractant chemok<strong>in</strong>e CXCL13.<br />
We further demonstrated that PP FDCs support<br />
preferential switch<strong>in</strong>g of activated B cells to<br />
IgA through abundant production of TGFβ<br />
(Figure 2).<br />
Recent publications<br />
Tsuji M, Suzuki K, K<strong>in</strong>oshita K.,<br />
Fagarasan S. Dynamic <strong>in</strong>teractions<br />
between bacteria and immune<br />
cells lead<strong>in</strong>g to <strong>in</strong>test<strong>in</strong>al IgA<br />
synthesis. Sem<strong>in</strong> Immunol. 2008<br />
<strong>in</strong> press.<br />
Ha SA, Tsuji M, Suzuki K, Meek<br />
B, Yasuda N, Kaisho T, Fagarasan<br />
S. Regulation of B1 cell migration<br />
by signals through Toll-like<br />
receptors. J Exp Med. 2006 Oct<br />
30;203(11):2541-50<br />
Suzuki K, Ha SA, Tsuji M,<br />
Fagarasan S. Intest<strong>in</strong>al IgA<br />
synthesis: A primitive form of<br />
adaptive immunity that regulates<br />
microbial communities <strong>in</strong> the gut.<br />
Sem<strong>in</strong> Immunol. 2006 Dec 8;<br />
Fagarasan S. Intest<strong>in</strong>al IgA<br />
synthesis: a primitive form of<br />
adaptive immunity that regulates<br />
microbial communities <strong>in</strong> the<br />
gut. Curr Top Microbiol Immunol.<br />
2006;308:137-53. Review.<br />
Suzuki K, Meek B, Doi Y, Honjo<br />
T, Fagarasan S.: Two dist<strong>in</strong>ctive<br />
pathways for recruitment of naive<br />
and primed IgM+ B cells to the<br />
gut lam<strong>in</strong>a propria Proc Natl<br />
Acad Sci U S A. 2005 Feb 15;102<br />
(7):2482-6.<br />
Figure 2. Role of TLR stimulation of<br />
FDCs <strong>in</strong> GC formation and<br />
IgA synthesis <strong>in</strong> the gut.<br />
FDCs located <strong>in</strong> the light<br />
zone of the PP follicles<br />
express TLRs. They<br />
respond to stimulation<br />
through TLRs by secret<strong>in</strong>g<br />
factors, such as like<br />
CXCL13, that promote<br />
migration and survival<br />
of B cells and CD40L<br />
express<strong>in</strong>g T cells (Follicular<br />
helper T FH cells). B cell-T<br />
cell <strong>in</strong>teractions through<br />
MHC II-TCR are critical for<br />
the GC reaction <strong>in</strong> PPs.<br />
Other factors secreted by<br />
FDCs, like TGFβ, facilitate<br />
preferential class switch<strong>in</strong>g<br />
of B cells from IgM to IgA.<br />
IgA B cells generated <strong>in</strong><br />
GC of PPs migrate to the<br />
lam<strong>in</strong>a propria, where they<br />
further differentiate <strong>in</strong>to<br />
IgA-secret<strong>in</strong>g cells. IgA<br />
transported and secreted<br />
<strong>in</strong>to the <strong>in</strong>test<strong>in</strong>al lumen<br />
prevents bacterial access<br />
to the epithelium.<br />
43