in vivo
in vivo
in vivo
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Recent publications<br />
Masuda K, Kakugawa K,<br />
Nakayama T, M<strong>in</strong>ato M,<br />
Katsura Y, Kawamoto H. T cell<br />
l<strong>in</strong>eage determ<strong>in</strong>ation precedes<br />
the <strong>in</strong>itiation of TCRb gene<br />
rearrangement. J. Immunol.<br />
179: 3699-3706, (2007)<br />
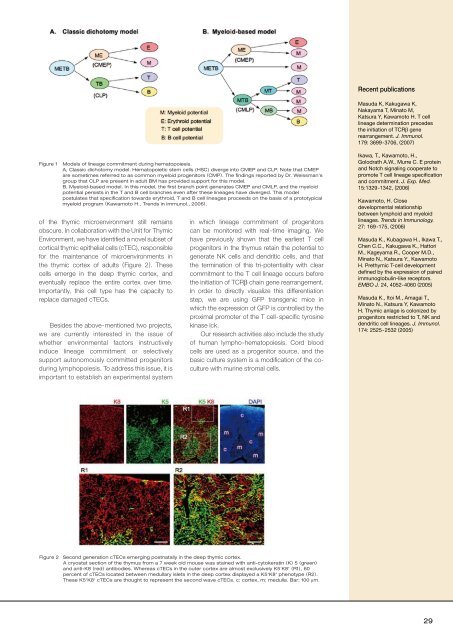
Figure 1<br />
Models of l<strong>in</strong>eage commitment dur<strong>in</strong>g hematopoiesis.<br />
A, Classic dichotomy model. Hematopoietic stem cells (HSC) diverge <strong>in</strong>to CMEP and CLP. Note that CMEP<br />
are sometimes referred to as common myeloid progenitors (CMP). The f<strong>in</strong>d<strong>in</strong>gs reported by Dr. Weissman’s<br />
group that CLP are present <strong>in</strong> adult BM has provided support for this model.<br />
B, Myeloid-based model. In this model, the first branch po<strong>in</strong>t generates CMEP and CMLP, and the myeloid<br />
potential persists <strong>in</strong> the T and B cell branches even after these l<strong>in</strong>eages have diverged. This model<br />
postulates that specification towards erythroid, T and B cell l<strong>in</strong>eages proceeds on the basis of a prototypical<br />
myeloid program (Kawamoto H., Trends <strong>in</strong> Immunol., 2006).<br />
of the thymic microenvironment still rema<strong>in</strong>s<br />
obscure. In collaboration with the Unit for Thymic<br />
Environment, we have identified a novel subset of<br />
cortical thymic epithelial cells (cTEC), responsible<br />
for the ma<strong>in</strong>tenance of microenvironments <strong>in</strong><br />
the thymic cortex of adults (Figure 2). These<br />
cells emerge <strong>in</strong> the deep thymic cortex, and<br />
eventually replace the entire cortex over time.<br />
Importantly, this cell type has the capacity to<br />
replace damaged cTECs.<br />
Besides the above-mentioned two projects,<br />
we are currently <strong>in</strong>terested <strong>in</strong> the issue of<br />
whether environmental factors <strong>in</strong>structively<br />
<strong>in</strong>duce l<strong>in</strong>eage commitment or selectively<br />
support autonomously committed progenitors<br />
dur<strong>in</strong>g lymphopoiesis. To address this issue, it is<br />
important to establish an experimental system<br />
<strong>in</strong> which l<strong>in</strong>eage commitment of progenitors<br />
can be monitored with real-time imag<strong>in</strong>g. We<br />
have previously shown that the earliest T cell<br />
progenitors <strong>in</strong> the thymus reta<strong>in</strong> the potential to<br />
generate NK cells and dendritic cells, and that<br />
the term<strong>in</strong>ation of this tri-potentiality with clear<br />
commitment to the T cell l<strong>in</strong>eage occurs before<br />
the <strong>in</strong>itiation of TCRβ cha<strong>in</strong> gene rearrangement.<br />
In order to directly visualize this differentiation<br />
step, we are us<strong>in</strong>g GFP transgenic mice <strong>in</strong><br />
which the expression of GFP is controlled by the<br />
proximal promoter of the T cell-specific tyros<strong>in</strong>e<br />
k<strong>in</strong>ase lck.<br />
Our research activities also <strong>in</strong>clude the study<br />
of human lympho-hematopoiesis. Cord blood<br />
cells are used as a progenitor source, and the<br />
basic culture system is a modification of the coculture<br />
with mur<strong>in</strong>e stromal cells.<br />
Ikawa, T., Kawamoto, H.,<br />
Golodrath A.W., Murre C. E prote<strong>in</strong><br />
and Notch signal<strong>in</strong>g cooperate to<br />
promote T cell l<strong>in</strong>eage specification<br />
and commitment. J. Exp. Med.<br />
15:1329-1342, (2006)<br />
Kawamoto, H. Close<br />
developmental relationship<br />
between lymphoid and myeloid<br />
l<strong>in</strong>eages. Trends <strong>in</strong> Immunology.<br />
27: 169-175, (2006)<br />
Masuda K., Kubagawa H., Ikawa T.,<br />
Chen C.C., Kakugawa K., Hattori<br />
M., Kageyama R., Cooper M.D.,<br />
M<strong>in</strong>ato N., Katsura Y., Kawamoto<br />
H. Prethymic T-cell development<br />
def<strong>in</strong>ed by the expression of paired<br />
immunoglobul<strong>in</strong>-like receptors.<br />
EMBO J. 24, 4052-4060 (2005)<br />
Masuda K., Itoi M., Amagai T.,<br />
M<strong>in</strong>ato N., Katsura Y, Kawamoto<br />
H. Thymic anlage is colonized by<br />
progenitors restricted to T, NK and<br />
dendritic cell l<strong>in</strong>eages. J. Immunol.<br />
174: 2525-2532 (2005)<br />
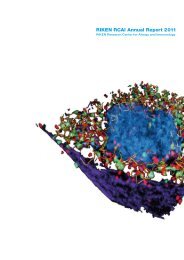
Figure 2 Second generation cTECs emerg<strong>in</strong>g postnatally <strong>in</strong> the deep thymic cortex.<br />
A cryostat section of the thymus from a 7 week old mouse was sta<strong>in</strong>ed with anti-cytokerat<strong>in</strong> (K) 5 (green)<br />
and anti-K8 (red) antibodies. Whereas cTECs <strong>in</strong> the outer cortex are almost exclusively K5 - K8 + (R1), 60<br />
percent of cTECs located between medullary islets <strong>in</strong> the deep cortex displayed a K5 + K8 + phenotype (R2).<br />
These K5 + K8 + cTECs are thought to represent the second wave cTECs. c: cortex, m: medulla. Bar; 100 mm.<br />
29