in vivo
in vivo
in vivo
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Recent publications<br />
DAPI<br />
dig-dUTP<br />
Np95<br />
Merge<br />
TKO+5me-dCTP<br />
Sharif J., Muto M., Takebayashi<br />
S., Suetake I., Iwamatsu A., Endo<br />
T.A., Sh<strong>in</strong>ga J., Mizutani-Koseki<br />
Y., Toyoda T., Okamura K., Tajima<br />
S., Mitsuya K., Okano M., Koseki<br />
H. (2007) The SRA prote<strong>in</strong> Np95<br />
mediates epigenetic <strong>in</strong>heritance<br />
by recruit<strong>in</strong>g Dnmt1 to methylated<br />
DNA. Nature 450:908-912.<br />
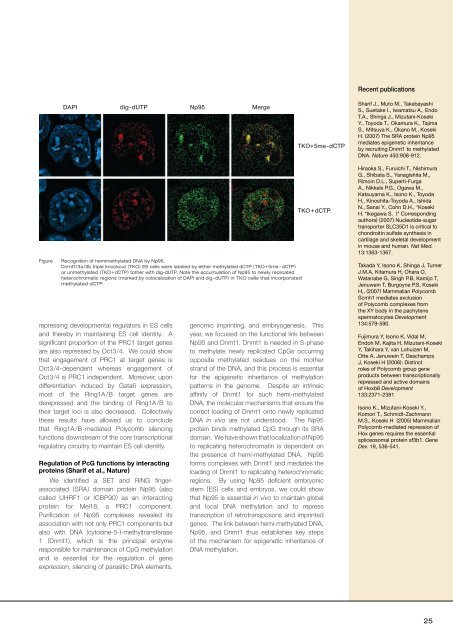
Figure<br />
Recognition of hemimethylated DNA by Np95.<br />
Dnmt1/3a/3b triple knockout (TKO) ES cells were labeled by either methylated dCTP (TKO+ 5me - dCTP)<br />
or unmethylated (TKO+ dCTP) tother with dig-dUTP. Note the accumulation of Np95 to newly replicated<br />
heterochromatic regions (marked by colocalization of DAPI and dig-dUTP) <strong>in</strong> TKO cells that <strong>in</strong>corporated<br />
methylated dCTP.<br />
repress<strong>in</strong>g developmental regulators <strong>in</strong> ES cells<br />
and thereby <strong>in</strong> ma<strong>in</strong>ta<strong>in</strong><strong>in</strong>g ES cell identity. A<br />
significant proportion of the PRC1 target genes<br />
are also repressed by Oct3/4. We could show<br />
that engagement of PRC1 at target genes is<br />
Oct3/4-dependent whereas engagement of<br />
Oct3/4 is PRC1 <strong>in</strong>dependent. Moreover, upon<br />
differentiation <strong>in</strong>duced by Gata6 expression,<br />
most of the R<strong>in</strong>g1A/B target genes are<br />
derepressed and the b<strong>in</strong>d<strong>in</strong>g of R<strong>in</strong>g1A/B to<br />
their target loci is also decreased. Collectively<br />
these results have allowed us to conclude<br />
that R<strong>in</strong>g1A/B-mediated Polycomb silenc<strong>in</strong>g<br />
functions downstream of the core transcriptional<br />
regulatory circuitry to ma<strong>in</strong>ta<strong>in</strong> ES cell identity.<br />
Regulation of PcG functions by <strong>in</strong>teract<strong>in</strong>g<br />
prote<strong>in</strong>s (Sharif et al., Nature)<br />
We identified a SET and RING f<strong>in</strong>gerassociated<br />
(SRA) doma<strong>in</strong> prote<strong>in</strong> Np95 (also<br />
called UHRF1 or ICBP90) as an <strong>in</strong>teract<strong>in</strong>g<br />
prote<strong>in</strong> for Mel18, a PRC1 component.<br />
Purification of Np95 complexes revealed its<br />
association with not only PRC1 components but<br />
also with DNA (cytos<strong>in</strong>e-5-)-methyltransferase<br />
1 (Dnmt1), which is the pr<strong>in</strong>cipal enzyme<br />
responsible for ma<strong>in</strong>tenance of CpG methylation<br />
and is essential for the regulation of gene<br />
expression, silenc<strong>in</strong>g of parasitic DNA elements,<br />
TKO+dCTP<br />
genomic impr<strong>in</strong>t<strong>in</strong>g, and embryogenesis. This<br />
year, we focused on the functional l<strong>in</strong>k between<br />
Np95 and Dnmt1. Dnmt1 is needed <strong>in</strong> S-phase<br />
to methylate newly replicated CpGs occurr<strong>in</strong>g<br />
opposite methylated residues on the mother<br />
strand of the DNA, and this process is essential<br />
for the epigenetic <strong>in</strong>heritance of methylation<br />
patterns <strong>in</strong> the genome. Despite an <strong>in</strong>tr<strong>in</strong>sic<br />
aff<strong>in</strong>ity of Dnmt1 for such hemi-methylated<br />
DNA, the molecular mechanisms that ensure the<br />
correct load<strong>in</strong>g of Dnmt1 onto newly replicated<br />
DNA <strong>in</strong> <strong>vivo</strong> are not understood. The Np95<br />
prote<strong>in</strong> b<strong>in</strong>ds methylated CpG through its SRA<br />
doma<strong>in</strong>. We have shown that localization of Np95<br />
to replicat<strong>in</strong>g heterochromat<strong>in</strong> is dependent on<br />
the presence of hemi-methylated DNA. Np95<br />
forms complexes with Dnmt1 and mediates the<br />
load<strong>in</strong>g of Dnmt1 to replicat<strong>in</strong>g heterochromatic<br />
regions. By us<strong>in</strong>g Np95 deficient embryonic<br />
stem (ES) cells and embryos, we could show<br />
that Np95 is essential <strong>in</strong> <strong>vivo</strong> to ma<strong>in</strong>ta<strong>in</strong> global<br />
and local DNA methylation and to repress<br />
transcription of retrotransposons and impr<strong>in</strong>ted<br />
genes. The l<strong>in</strong>k between hemi-methylated DNA,<br />
Np95, and Dnmt1 thus establishes key steps<br />
of the mechanism for epigenetic <strong>in</strong>heritance of<br />
DNA methylation.<br />
Hiraoka S., Furuichi T., Nishimura<br />
G., Shibata S., Yanagishita M.,<br />
Rimo<strong>in</strong> D.L., Superti-Furga<br />
A., Nikkels P.G., Ogawa M.,<br />
Katsuyama K., Isono K., Toyoda<br />
H., K<strong>in</strong>oshita-Toyoda A., Ishida<br />
N., Sanai Y., Cohn D.H., *Koseki<br />
H. *Ikegawa S. (* Correspond<strong>in</strong>g<br />
authors) (2007) Nucleotide-sugar<br />
transporter SLC35D1 is critical to<br />
chondroit<strong>in</strong> sulfate synthesis <strong>in</strong><br />
cartilage and skeletal development<br />
<strong>in</strong> mouse and human. Nat Med.<br />
13:1363-1367.<br />
Takada Y, Isono K, Sh<strong>in</strong>ga J, Turner<br />
J.M.A, Kitamura H, Ohara O,<br />
Watanabe G, S<strong>in</strong>gh P.B, Kamijo T,<br />
Jenuwe<strong>in</strong> T, Burgoyne P.S, Koseki<br />
H,, (2007) Mammalian Polycomb<br />
Scmh1 mediates exclusion<br />
of Polycomb complexes from<br />
the XY body <strong>in</strong> the pachytene<br />
spermatocytes Development<br />
134:579-590.<br />
Fujimura Y, Isono K, Vidal M,<br />
Endoh M, Kajita H, Mizutani-Koseki<br />
Y, Takihara Y, van Lohuizen M,<br />
Otte A, Jenuwe<strong>in</strong> T, Deschamps<br />
J, Koseki H (2006): Dist<strong>in</strong>ct<br />
roles of Polycomb group gene<br />
products between transcriptionally<br />
repressed and active doma<strong>in</strong>s<br />
of Hoxb8 Development<br />
133:2371-2381<br />
Isono K., Mizutani-Koseki Y.,<br />
Komori T., Schmidt-Zachmann<br />
M.S., Koseki H (2005) Mammalian<br />
Polycomb-mediated repression of<br />
Hox genes requires the essential<br />
spliceosomal prote<strong>in</strong> sf3b1. Gene<br />
Dev. 19, 536-541.<br />
25