Molluscan Research: Techniques for collecting, handling, preparing ...
Molluscan Research: Techniques for collecting, handling, preparing ...
Molluscan Research: Techniques for collecting, handling, preparing ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
16<br />
Chemical deterioration<br />
Byne’s ‘disease’<br />
Micromolluscs are often fragile and prone to adverse<br />
reaction with acids and salts. A serious destructive effect on<br />
calcium carbonate shells is known as ‘Byne’s disease’,<br />
named <strong>for</strong> L. St. G. Byne (1872–1947) a British amateur<br />
shell collector who first described the phenomenon (Byne<br />
1899a, b). Its manifestation is a white efflorescence covering<br />
the shell, which eventually destroys the specimen<br />
completely. Byne’s explanation that it was caused by butyric<br />
and acetic acid was partly correct. It seems not to be caused<br />
by shells with remaining animal tissue since the old<br />
collections where it occurs often contain empty shells only<br />
(pers. obs. by the authors). Tennent and Baird (1985)<br />
identified the crystalline substance as a mixture of calcium<br />
acetate and <strong>for</strong>miate and considered that the acetic and<br />
<strong>for</strong>mic acid originated from the oak-wood frequently used in<br />
cabinets. The reaction can also be accelerated by low air<br />
circulation and by high humidity, where the water molecules<br />
act as an extractor and carrier <strong>for</strong> the acids. The necessity <strong>for</strong><br />
well aerated cabinets was pointed out as a precaution by<br />
Byne (1899a, b). Some other organic substances are also<br />
likely culprits and include cork, natural cotton, fibre-wood or<br />
particle board, where <strong>for</strong>maldehyde-based glues are used, as<br />
well as any surface treatment evaporating <strong>for</strong>malin as<br />
applied <strong>for</strong> instance to some metal cabinets. All of these<br />
substances should be avoided. A number of articles have<br />
been written on various aspects of Byne’s disease (Kenyon<br />
1909; Lamy 1933; Nicholls 1934; Nockert and Wadsten<br />
1978; Padfield et al. 1982; Grosjean and Fung 1984;<br />
Hatchfield and Carpenter 1985; Kolff 1988; Kamath et al.<br />
1985; Davies 1987; Davies 1988; Hertz 1990; Pinto de<br />
Oliveria and de Cassia da Silveira e Sa 1996; Stamol 1998;<br />
Callomon 2000, 2003; de Prins 2005).<br />
Glass ‘disease’<br />
As in Byne’s disease, the so called ‘glass disease’<br />
produces an efflorescence of calcium salts and, unless halted,<br />
will lead to complete destruction of specimens in as little as<br />
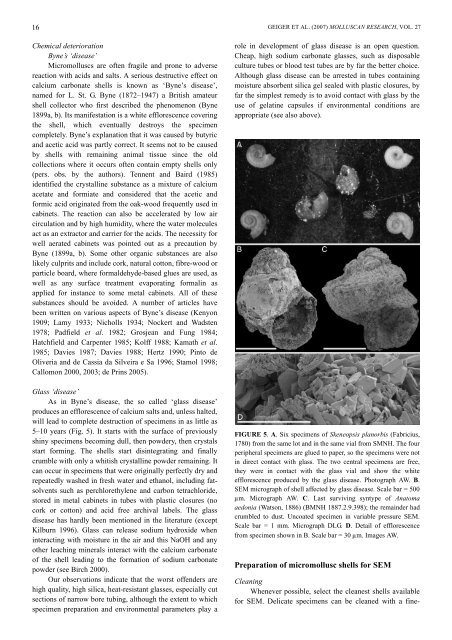
5–10 years (Fig. 5). It starts with the surface of previously<br />
shiny specimens becoming dull, then powdery, then crystals<br />
start <strong>for</strong>ming. The shells start disintegrating and finally<br />
crumble with only a whitish crystalline powder remaining. It<br />
can occur in specimens that were originally perfectly dry and<br />
repeatedly washed in fresh water and ethanol, including fatsolvents<br />
such as perchlorethylene and carbon tetrachloride,<br />
stored in metal cabinets in tubes with plastic closures (no<br />
cork or cotton) and acid free archival labels. The glass<br />
disease has hardly been mentioned in the literature (except<br />
Kilburn 1996). Glass can release sodium hydroxide when<br />
interacting with moisture in the air and this NaOH and any<br />
other leaching minerals interact with the calcium carbonate<br />
of the shell leading to the <strong>for</strong>mation of sodium carbonate<br />
powder (see Birch 2000).<br />
Our observations indicate that the worst offenders are<br />
high quality, high silica, heat-resistant glasses, especially cut<br />
sections of narrow bore tubing, although the extent to which<br />
specimen preparation and environmental parameters play a<br />
GEIGER ET AL. (2007) MOLLUSCAN RESEARCH, VOL. 27<br />
role in development of glass disease is an open question.<br />
Cheap, high sodium carbonate glasses, such as disposable<br />
culture tubes or blood test tubes are by far the better choice.<br />
Although glass disease can be arrested in tubes containing<br />
moisture absorbent silica gel sealed with plastic closures, by<br />
far the simplest remedy is to avoid contact with glass by the<br />
use of gelatine capsules if environmental conditions are<br />
appropriate (see also above).<br />
FIGURE 5. A. Six specimens of Skeneopsis planorbis (Fabricius,<br />
1780) from the same lot and in the same vial from SMNH. The four<br />
peripheral specimens are glued to paper, so the specimens were not<br />
in direct contact with glass. The two central specimens are free,<br />
they were in contact with the glass vial and show the white<br />
efflorescence produced by the glass disease. Photograph AW. B.<br />
SEM micrograph of shell affected by glass disease. Scale bar = 500<br />
µm. Micrograph AW. C. Last surviving syntype of Anatoma<br />
aedonia (Watson, 1886) (BMNH 1887.2.9.398); the remainder had<br />
crumbled to dust. Uncoated specimen in variable pressure SEM.<br />
Scale bar = 1 mm. Micrograph DLG. D. Detail of efflorescence<br />
from specimen shown in B. Scale bar = 30 µm. Images AW.<br />
Preparation of micromollusc shells <strong>for</strong> SEM<br />
Cleaning<br />
Whenever possible, select the cleanest shells available<br />
<strong>for</strong> SEM. Delicate specimens can be cleaned with a fine-