Molluscan Research: Techniques for collecting, handling, preparing ...
Molluscan Research: Techniques for collecting, handling, preparing ...
Molluscan Research: Techniques for collecting, handling, preparing ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
28<br />
slowly rotate the column bottom while carefully pulling the<br />
filter from underneath the retention ring from one segment<br />
after the other. Wash the radula in water and mount. Retrieval<br />
GEIGER ET AL. (2007) MOLLUSCAN RESEARCH, VOL. 27<br />
rate is approximately 80–90%. Proteinase K, though, does<br />
not work well on <strong>for</strong>malin fixed material, in which proteins<br />
of the tissue have been cross-linked (Holznagel 1998).<br />
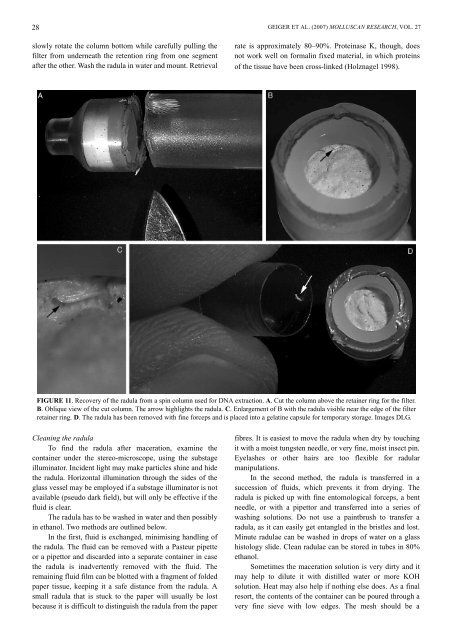
FIGURE 11. Recovery of the radula from a spin column used <strong>for</strong> DNA extraction. A. Cut the column above the retainer ring <strong>for</strong> the filter.<br />
B. Oblique view of the cut column. The arrow highlights the radula. C. Enlargement of B with the radula visible near the edge of the filter<br />
retainer ring. D. The radula has been removed with fine <strong>for</strong>ceps and is placed into a gelatine capsule <strong>for</strong> temporary storage. Images DLG.<br />
Cleaning the radula<br />
To find the radula after maceration, examine the<br />
container under the stereo-microscope, using the substage<br />
illuminator. Incident light may make particles shine and hide<br />
the radula. Horizontal illumination through the sides of the<br />
glass vessel may be employed if a substage illuminator is not<br />
available (pseudo dark field), but will only be effective if the<br />
fluid is clear.<br />
The radula has to be washed in water and then possibly<br />
in ethanol. Two methods are outlined below.<br />
In the first, fluid is exchanged, minimising <strong>handling</strong> of<br />
the radula. The fluid can be removed with a Pasteur pipette<br />
or a pipettor and discarded into a separate container in case<br />
the radula is inadvertently removed with the fluid. The<br />
remaining fluid film can be blotted with a fragment of folded<br />
paper tissue, keeping it a safe distance from the radula. A<br />
small radula that is stuck to the paper will usually be lost<br />
because it is difficult to distinguish the radula from the paper<br />
fibres. It is easiest to move the radula when dry by touching<br />
it with a moist tungsten needle, or very fine, moist insect pin.<br />
Eyelashes or other hairs are too flexible <strong>for</strong> radular<br />
manipulations.<br />
In the second method, the radula is transferred in a<br />
succession of fluids, which prevents it from drying. The<br />
radula is picked up with fine entomological <strong>for</strong>ceps, a bent<br />
needle, or with a pipettor and transferred into a series of<br />
washing solutions. Do not use a paintbrush to transfer a<br />
radula, as it can easily get entangled in the bristles and lost.<br />
Minute radulae can be washed in drops of water on a glass<br />
histology slide. Clean radulae can be stored in tubes in 80%<br />
ethanol.<br />
Sometimes the maceration solution is very dirty and it<br />
may help to dilute it with distilled water or more KOH<br />
solution. Heat may also help if nothing else does. As a final<br />
resort, the contents of the container can be poured through a<br />
very fine sieve with low edges. The mesh should be a