The Ecology of Tidal Freshwater Marshes of the - USGS National ...
The Ecology of Tidal Freshwater Marshes of the - USGS National ...
The Ecology of Tidal Freshwater Marshes of the - USGS National ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
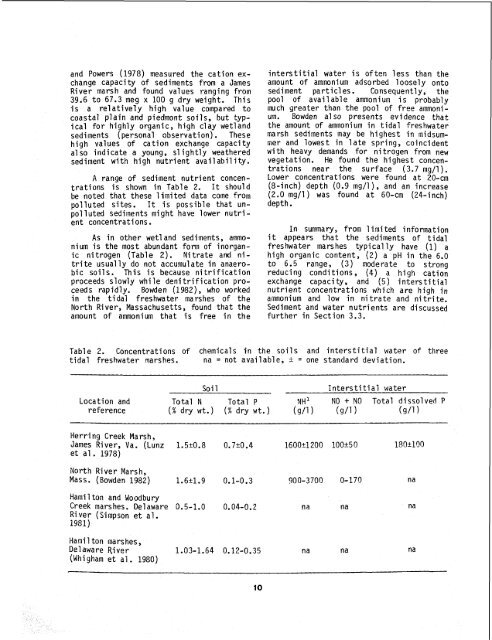
and Powers (1978) measured <strong>the</strong> cation exchangecapacity <strong>of</strong> sediments from a JamesRiver marsh and found values ranging from39.6 to 67.3 meg x 100 g dry weight. Thisis a relatively high value compared tocoastal plain and piedmont soils, but typicalfor highly organic, high clay wetlandsediments (personal observation). <strong>The</strong>sehigh values <strong>of</strong> cation exchange capacitya1 so indicate a young, sl ightly wea<strong>the</strong>redsediment with high nutrient avail abil ity.A range <strong>of</strong> sediment nutrient concentrationsis shown in Table 2. It shouldbe noted that <strong>the</strong>se limited data come frompolluted sites. It is possible that unpollutedsediments might have lower nutrientconcentrations.As in o<strong>the</strong>r wet1 and sediments, ammoniumis <strong>the</strong> most abundant form <strong>of</strong> inorganicnitrogen (Table 2). Nitrate and nitriteusually do not accumulate in anaerobicsoils. This is because nitrificationproceeds slowly while deni trification proceedsrapidly. Bowden (1982), who workedin <strong>the</strong> tidal freshwater marshes <strong>of</strong> <strong>the</strong>North River, Massachusetts, found that <strong>the</strong>amount <strong>of</strong> ammonium that is free in <strong>the</strong>interstitial water is <strong>of</strong>ten less than <strong>the</strong>amount <strong>of</strong> ammonium adsorbed loosely ontosediment particles. Consequently, <strong>the</strong>pool <strong>of</strong> available ammonium is probablymuch greater than <strong>the</strong> pool <strong>of</strong> free ammonium.Bowden also presents evidence that<strong>the</strong> amount <strong>of</strong> ammonium in tidal freshwatermarsh sediments may be highest in midsummerand lowest in late spring, coincidentwith heavy demands for nitrogen from newvegetation. He found <strong>the</strong> highest concentrationsnear <strong>the</strong> surface (3.7 mg/l).Lower concentrations were found at 20-cm(8-inch) depth (0.9 mg/l), and an increase(2.0 mg/l ) was found at 60-cm (24-inch)depth.In summary, from 1 imi ted informationit appears that <strong>the</strong> sediments <strong>of</strong> tidalfreshwater marshes typically have (1) ahigh organic content, (2) a pH in <strong>the</strong> 6.0to 6.5 range, (3) moderate to strongreducing conditions, (4) a high cationexchange capacity, and (5) inters ti tialnutrient concentrations which are high inaiamonium and low in nitrate and nitrite.Sediment and water nutrients are discussedfur<strong>the</strong>r in Section 3.3.Table 2. Concentrations <strong>of</strong> chemicals in <strong>the</strong> soils and interstitial water <strong>of</strong> threetidal freshwater marshes. na = not available, + = one standard deviation.Soi 1Interstitial waterLocation and Total N Total P NH NO + NO Total dissolved Preference (% dry wt.) (% dry wt.) (g/l) (9/1) (9/1)Herring Creek Marsh,James River, Va. (Lunz 1.5r0.8 0.7k0.4 1600t1200 look50 180k190et a1 . 1978)North River Marsh,Mass. (Bowden 1982) 1.6r1.9 0.1-0.3 900-3700 0-170 n aHami 1 ton and WoodburyCreek marshes. Delaware 0.5-1.0 0.04-0.2 na naRiver (Simpson et al.1981)Hami 1 ton marshes ,Delaware River(Whi gham et a1 . 1980)1.03-1.64 0.12-0.35 n a na