Organic Chemistry Structures of Organic Compounds
Organic Chemistry Structures of Organic Compounds
Organic Chemistry Structures of Organic Compounds
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
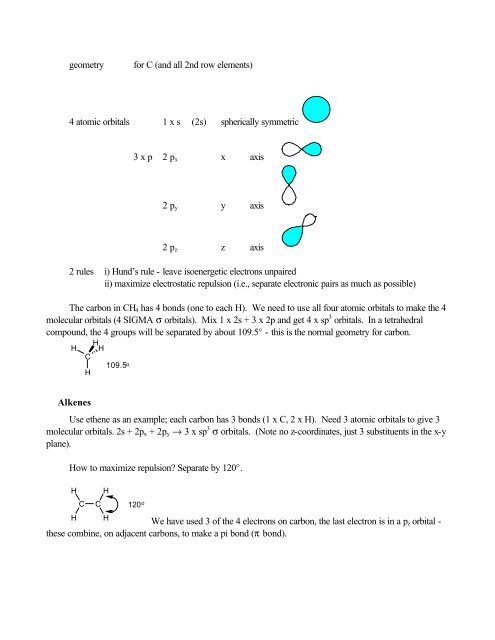
geometryfor C (and all 2nd row elements)4 atomic orbitals 1 x s (2s) spherically symmetric3 x p 2 p x x axis2 p y y axis2 p z z axis2 rules i) Hund’s rule - leave isoenergetic electrons unpairedii) maximize electrostatic repulsion (i.e., separate electronic pairs as much as possible)The carbon in CH 4 has 4 bonds (one to each H). We need to use all four atomic orbitals to make the 4molecular orbitals (4 SIGMA σ orbitals). Mix 1 x 2s + 3 x 2p and get 4 x sp 3 orbitals. In a tetrahedralcompound, the 4 groups will be separated by about 109.5° - this is the normal geometry for carbon.HHHC109.5 oHAlkenesUse ethene as an example; each carbon has 3 bonds (1 x C, 2 x H). Need 3 atomic orbitals to give 3molecular orbitals. 2s + 2p x + 2p y → 3 x sp 3 σ orbitals. (Note no z-coordinates, just 3 substituents in the x-yplane).How to maximize repulsion? Separate by 120°.HHCCHH120 o We have used 3 <strong>of</strong> the 4 electrons on carbon, the last electron is in a p z orbital -these combine, on adjacent carbons, to make a pi bond (π bond).