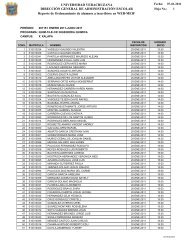
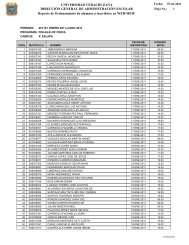
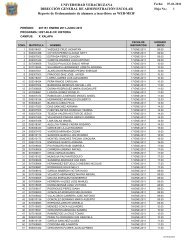
Understanding the Software Options
Understanding the Software Options
Understanding the Software Options
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Solids Processing<br />
FIGURE 4. (Top left) In <strong>the</strong> test for “aerated” conditions, air<br />
�ows through <strong>the</strong> sample from top to bottom, which are both<br />
closed by sintered glass discs. The cylindrical section has a 50<br />
mm dia. and a height of 80 mm. (Bottom) For "air over layer"<br />
testing, warm air �ows over <strong>the</strong> powder layer in <strong>the</strong> sample<br />
tray. (Top right) The wire basket for “basket testing” is illustrated<br />
more clearly in Figure 5<br />
heat generation rate, increases exponentially<br />
with temperature. Consequently,<br />
<strong>the</strong> heat generation rate will<br />
exceed <strong>the</strong> rate of heat loss and <strong>the</strong><br />
temperature of <strong>the</strong> material will rise<br />
higher. This process is referred to as<br />
self-heating. Self-heating begins at a<br />
temperature at which <strong>the</strong> rate of heat<br />
generation is greater than <strong>the</strong> rate of<br />
heat loss and this temperature is called<br />
<strong>the</strong> exo<strong>the</strong>rmic onset temperature.<br />
Subsequent effects<br />
Potential smoldering. Self-heating<br />
of solid materials usually results in<br />
smoldering, which can set <strong>the</strong> material<br />
on fire or cause dust explosions,<br />
particularly when <strong>the</strong> smoldering<br />
material is disturbed and exposed to<br />
air (Figure 1). Many plants that experience<br />
self-heating incidents have<br />
a history of “near misses” where some<br />
self-heating occurs but does not progress<br />
to full-blown ignition. In such<br />
cases <strong>the</strong>re may be “black spots” in an<br />
o<strong>the</strong>rwise light-colored product, or a<br />
lump of charred product may be found,<br />
a so-called “smoldering nest”. It is important<br />
to recognize such occurrences<br />
as indications of a potentially serious<br />
problem, ra<strong>the</strong>r than to learn to live<br />
with it.<br />
Potential release of flammable<br />
46 CHEMICAL ENGINEERING WWW.CHE.COM AUGUST 2011<br />
gases. Self-heating reactions may<br />
also produce flammable gases, which<br />
may lead to gas explosions in process<br />
vessels or compromise product quality<br />
(Figure 2).<br />
Testing self-heating behavior<br />
The exo<strong>the</strong>rmic onset temperature<br />
is influenced not only by <strong>the</strong> chemical<br />
and physical properties, such as<br />
chemical reaction kinetics and heat<br />
of reaction, but also by o<strong>the</strong>r factors,<br />
including <strong>the</strong> following:<br />
• Dimension and geometry of <strong>the</strong> solid<br />
bulk<br />
• Ambient airflow<br />
• Availability of oxygen in <strong>the</strong> bulk<br />
• Additives and contaminants<br />
Usually, <strong>the</strong> material has to be exposed<br />
to an elevated temperature for a period<br />
of time before it self-heats. This time is<br />
referred to as <strong>the</strong> induction time, which<br />
is dependent on temperature; and usually<br />
<strong>the</strong> higher <strong>the</strong> temperature, <strong>the</strong><br />
shorter <strong>the</strong> induction time will be.<br />
Because of <strong>the</strong> influencing factors<br />
mentioned, a single test is usually unable<br />
to predict self-heating behavior<br />
for all different drying and storage<br />
conditions. Instead, separate tests<br />
have been developed to simulate <strong>the</strong><br />
conditions where <strong>the</strong> powder is in bulk<br />
form (Figure 3), layer form (with air<br />
FIGURE 5. “Basket test” sample holders, for testing at different<br />
scales, allow extrapolation to large-scale storage conditions.<br />
The baskets typically have sides of 25, 50 and 100 mm<br />
FIGURE 6. This “basket test” sample holder is prepared for<br />
testing inside a laboratory oven<br />
flowing over <strong>the</strong> powder; Figure 4)<br />
and aerated form (Figure 4), where<br />
air is passing through <strong>the</strong> bulk of <strong>the</strong><br />
product, increasing <strong>the</strong> oxygen availability<br />
for <strong>the</strong> reaction and also helping<br />
to remove heat from <strong>the</strong> reacting<br />
material. For large-scale storage situations<br />
tests are carried out at different<br />
scales so that <strong>the</strong> effect of <strong>the</strong> size<br />
of <strong>the</strong> bulk material can be assessed<br />
(Figure 5). All tests are carried out in<br />
temperature-controlled ovens (Figure<br />
6) that allow screening tests (with <strong>the</strong><br />
temperature ramped up at a defined<br />
rate) and iso<strong>the</strong>rmal testing (with<br />
a constant temperature controlled<br />
within narrow margins). Because of<br />
<strong>the</strong> potential for violent reactions during<br />
<strong>the</strong> self-heating process, all testing<br />
equipment needs to be fitted with explosion<br />
protection.<br />
Learning from a real incident<br />
In one incident, <strong>the</strong> powder in a fluidized<br />
bed dryer caught fire when <strong>the</strong><br />
powder conveyer in <strong>the</strong> dryer was<br />
turned off in order to fix clogging in<br />
an upstream wet-product conveyer.<br />
During this period, <strong>the</strong> hot air supply<br />
was continued.<br />
A screening test was conducted to<br />
determine whe<strong>the</strong>r this powder could<br />
self-heat under <strong>the</strong> conditions that ex-