Understanding the Software Options
Understanding the Software Options
Understanding the Software Options
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Temperature, °C<br />
Self-heating test with a constant rate<br />
of temperature rise, 0.5°C/min<br />
450<br />
400<br />
350<br />
Oven<br />
75% height<br />
300<br />
50% height<br />
250<br />
200<br />
150<br />
100<br />
50<br />
25% height<br />
12.5% height<br />
0<br />
0 100 200 300 400 500 600 700<br />
Time, min<br />
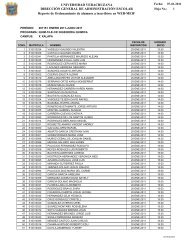
FIGURE 7. In this screening test, <strong>the</strong> oven temperature was<br />
increased from 20 to 400°C at a rate of 0.5°C/min. The exo<strong>the</strong>rmic<br />
onset temperature was identi�ed to be 166°C, which was lower<br />
than <strong>the</strong> hot air temperature of <strong>the</strong> dryer, indicating that selfheating<br />
was <strong>the</strong> most probable ignition source for this incident<br />
isted in <strong>the</strong> dryer before <strong>the</strong> incident.<br />
An typical powder sample was placed<br />
in a temperature-programmed oven<br />
and <strong>the</strong> temperature of <strong>the</strong> oven was<br />
increased from 20 to 400°C at a rate<br />
of 0.5°C/min (Figure 7). The exo<strong>the</strong>rmic<br />
onset temperature was identified<br />
to be 166°C, which was lower than <strong>the</strong><br />
hot air temperature of <strong>the</strong> dryer. The<br />
result indicated that self-heating was<br />
<strong>the</strong> most probable ignition source for<br />
this incident.<br />
This test provides a useful tool for a<br />
quick identification of <strong>the</strong> self-ignition<br />
hazard of materials and should be conducted<br />
for materials whose <strong>the</strong>rmal<br />
stability characteristics are not known.<br />
However, to be of any practical value,<br />
<strong>the</strong> test results obtained in a laboratory-scale<br />
apparatus have to be scaled<br />
up to plant-size process. In order to<br />
establish <strong>the</strong> relationship between<br />
<strong>the</strong> exo<strong>the</strong>rmic onset temperature and<br />
powder layer thickness with <strong>the</strong> aid of<br />
<strong>the</strong>rmal explosion <strong>the</strong>ory, <strong>the</strong> sample of<br />
<strong>the</strong> powder that was being dried in <strong>the</strong><br />
same incident batch was tested at three<br />
different layer thicknesses. In each<br />
trial, <strong>the</strong> powder sample was exposed<br />
to a constant temperature to test if<br />
self-heating would actually take place.<br />
The highest temperature at which selfheating<br />
did not occur and <strong>the</strong> lowest<br />
temperature at which self-heating did<br />
occur were determined. The average<br />
value of <strong>the</strong>se two temperatures was<br />
taken as <strong>the</strong> exo<strong>the</strong>rmic onset temperature<br />
for each powder layer.<br />
The exo<strong>the</strong>rmic onset temperatures<br />
were used to determine <strong>the</strong><br />
unknown constants of <strong>the</strong> following<br />
equation, which expresses <strong>the</strong> relationship<br />
between <strong>the</strong> exo<strong>the</strong>rmic<br />
onset temperature and <strong>the</strong> thickness<br />
of <strong>the</strong> powder layer:<br />
Onset temperature of self-heating, °C<br />
180<br />
170<br />
160<br />
150<br />
140<br />
130<br />
(1)<br />
Where<br />
Ta = Exo<strong>the</strong>rmic onset temperature<br />
for a powder layer, K<br />
r = One half of <strong>the</strong> powder layer<br />
thickness, m<br />
δc = Frank-Kamenetskii-parameter,<br />
dimensionless<br />
M, N = Constants determined by properties<br />
of <strong>the</strong> powder material<br />
Using Equation (1), <strong>the</strong> exo<strong>the</strong>rmic<br />
onset temperatures of layers of <strong>the</strong><br />
powder at different thicknesses were<br />
calculated and <strong>the</strong> results are plotted<br />
(Figure 8). As <strong>the</strong> thickness of <strong>the</strong><br />
powder layer was increased from 1 to<br />
12 in., <strong>the</strong> exo<strong>the</strong>rmic onset temperature<br />
decreased by 48°C.<br />
The powder layer in <strong>the</strong> dryer before<br />
<strong>the</strong> incident was on <strong>the</strong> order of 4–8<br />
in. This suggests that <strong>the</strong> exo<strong>the</strong>rmic<br />
onset temperature for <strong>the</strong> powder layer<br />
was well below <strong>the</strong> hot air temperature.<br />
Ignition would occur if <strong>the</strong> heating time<br />
exceeded <strong>the</strong> induction time.<br />
Concluding remarks<br />
The self-heating hazard of solid materials<br />
to be dried should be determined.<br />
Depending on <strong>the</strong> drying process, <strong>the</strong><br />
solid materials can be tested in different<br />
shape, heating environment, with<br />
or without an airflow through <strong>the</strong> material<br />
and an airflow at <strong>the</strong> surface of<br />
<strong>the</strong> material. The exo<strong>the</strong>rmic onset<br />
temperatures can be used to determine<br />
safe drying temperatures using<br />
sufficient safety margins. However, <strong>the</strong><br />
relationship between <strong>the</strong> exo<strong>the</strong>rmic<br />
onset temperature and <strong>the</strong> dimension<br />
of solid bulk is often needed in order to<br />
design a safe drying process. This rela-<br />
Dependence of self-heating onset<br />
on <strong>the</strong> thickness of powder layer<br />
120<br />
0 2 4 6 8 10 12<br />
Thickness of powder layer, in<br />
FIGURE 8. Using Equation (1), <strong>the</strong> exo<strong>the</strong>rmic onset temperatures<br />
of layers of <strong>the</strong> powder at different thicknesses were<br />
calculated and <strong>the</strong> results plotted here. As <strong>the</strong> thickness of <strong>the</strong><br />
powder layer was increased from 1 to 12 in., <strong>the</strong> exo<strong>the</strong>rmic<br />
onset temperature decreased by 48°C<br />
tionship can be established from selfheating<br />
experiments based on <strong>the</strong>rmal<br />
explosion <strong>the</strong>ories, as demonstrated by<br />
<strong>the</strong> example introduced above. ■<br />
Edited by Rebekkah Marshall<br />
Suggested reading<br />
J.A. Abbott (technical ed.), “Prevention of Fire &<br />
Explosions in Dryers”, 2nd ed., The Institution of<br />
Chemical Engineers, Rugby U.K. 1990.<br />
Authors<br />
Vahid Ebadat is <strong>the</strong> CEO<br />
of Chilworth North America<br />
(Chilworth Global, Princeton,<br />
NJ; Phone: 609-799-<br />
4449; Email: safety-usa@<br />
chilworthglobal.com; Website:<br />
www.chilworth.com). He holds<br />
a B.S. in electrical engineering<br />
and a Ph.D. from Southampton<br />
University. He has worked<br />
extensively as a process and<br />
operational hazards consultant<br />
for <strong>the</strong> chemical, pharmaceutical and food<br />
industries. Ebadat is a regular speaker at training<br />
courses on gas and vapor flammability, dust<br />
explosions, and controlling electrostatic hazards.<br />
He is a member of NFPA 77 Technical Committee<br />
on Static Electricity; NFPA 654 Standard for<br />
<strong>the</strong> Prevention of Fire and Dust Explosions from<br />
<strong>the</strong> Manufacturing, Processing, and Handling of<br />
Combustible Particular Solids; and ASTM E27<br />
Committee on Hazard Potential of Chemicals.<br />
Ebadat’s research has culminated in <strong>the</strong> publication<br />
of numerous technical articles and papers.<br />
Pieter Zeeuwen, is a senior<br />
process safety specialist at<br />
Chilworth Global. He holds<br />
a M.Sc. in applied physics<br />
from Eindhoven University<br />
of Technology and has more<br />
than 30 years experience<br />
in <strong>the</strong> gas and dust explosion<br />
fields, including materials<br />
testing, small and large<br />
scale explosion research, and<br />
consultancy for industry and<br />
government agencies in a number of countries.<br />
His areas of expertise include gas and dust explosion<br />
hazard assessment, gas and dust explosion<br />
prevention and protection, electrostatic hazard<br />
assessment, hazardous area classification, and<br />
gas cloud explosions as well as incident investigations.<br />
Over <strong>the</strong> years, Zeeuwen has served on<br />
many working groups including various standards<br />
committees, both nationally and internationally,<br />
for instance, most recently CEN (European<br />
Standards Committee) working groups on explosion<br />
protection methods and on test methods. He<br />
regularly lectures on various aspects of explosion<br />
safety and acts as seminar chairman and course<br />
director. Zeeuwen has published numerous articles<br />
in scientific journals and presented many<br />
papers at international conferences.<br />
CHEMICAL ENGINEERING WWW.CHE.COM AUGUST 2011 47