Mass Transfer & Porous Media (MTPM) - Andra
Mass Transfer & Porous Media (MTPM) - Andra
Mass Transfer & Porous Media (MTPM) - Andra
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
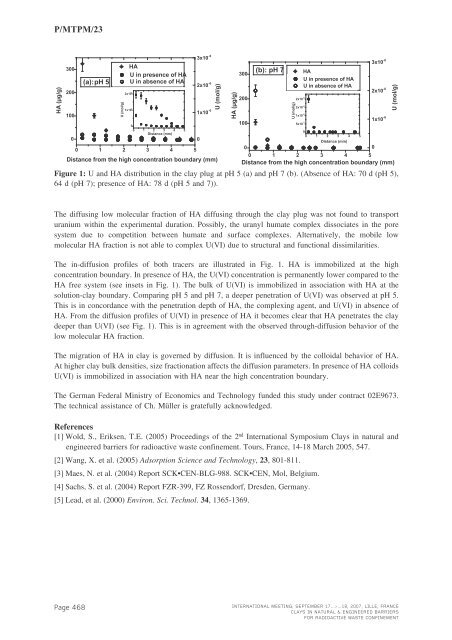
P/<strong>MTPM</strong>/23HA (µg/g)3002001000(a):pH 5U (mol/g)HAU in presence of HAU in absence of HA2x10 -71x10 -700 1 2 3 4 5Distance (mm)0 1 2 3 4 53x10 -62x10 -61x10 -6Distance from the high concentration boundary (mm)0U (mol/g)Figure 1: U and HA distribution in the clay plug at pH 5 (a) and pH 7 (b). (Absence of HA: 70 d (pH 5),64 d (pH 7); presence of HA: 78 d (pH 5 and 7)).HA (µg/g)3002001000(b): pH 7U (mol/g)2x10 -62x10 -61x10 -65x10 -7HAU in presence of HAU in absence of HA00 1 2 3 4 5Distance (mm)3x10 -62x10 -61x10 -60 1 2 3 4 5Distance from the high concentration boundary (mm)0U (mol/g)The diffusing low molecular fraction of HA diffusing through the clay plug was not found to transporturanium within the experimental duration. Possibly, the uranyl humate complex dissociates in the poresystem due to competition between humate and surface complexes. Alternatively, the mobile lowmolecular HA fraction is not able to complex U(VI) due to structural and functional dissimilarities.The in-diffusion profiles of both tracers are illustrated in Fig. 1. HA is immobilized at the highconcentration boundary. In presence of HA, the U(VI) concentration is permanently lower compared to theHA free system (see insets in Fig. 1). The bulk of U(VI) is immobilized in association with HA at thesolution-clay boundary. Comparing pH 5 and pH 7, a deeper penetration of U(VI) was observed at pH 5.This is in concordance with the penetration depth of HA, the complexing agent, and U(VI) in absence ofHA. From the diffusion profiles of U(VI) in presence of HA it becomes clear that HA penetrates the claydeeper than U(VI) (see Fig. 1). This is in agreement with the observed through-diffusion behavior of thelow molecular HA fraction.The migration of HA in clay is governed by diffusion. It is influenced by the colloidal behavior of HA.At higher clay bulk densities, size fractionation affects the diffusion parameters. In presence of HA colloidsU(VI) is immobilized in association with HA near the high concentration boundary.The German Federal Ministry of Economics and Technology funded this study under contract 02E9673.The technical assistance of Ch. Müller is gratefully acknowledged.References[1] Wold, S., Eriksen, T.E. (2005) Proceedings of the 2 nd International Symposium Clays in natural andengineered barriers for radioactive waste confinement. Tours, France, 14-18 March 2005, 547.[2] Wang, X. et al. (2005) Adsorption Science and Technology , 23, 801-811.[3] Maes, N. et al. (2004) Report SCK•CEN-BLG-988. SCK•CEN, Mol, Belgium.[4] Sachs, S. et al. (2004) Report FZR-399, FZ Rossendorf, Dresden, Germany.[5] Lead, et al. (2000) Environ. Sci. Technol. 34 , 1365-1369.Page 468INTERNATIONAL MEETING, SEPTEMBER 17...>...18, 2007, LILLE, FRANCECLAYS IN NATURAL & ENGINEERED BARRIERSFOR RADIOACTIVE WASTE CONFINEMENT