Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
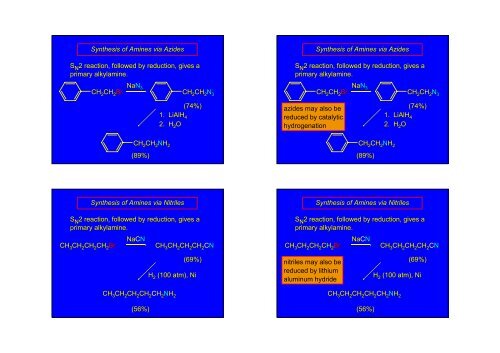
Synthesis of <strong>Amines</strong> via AzidesS N 2 reaction, followed by reduction, gives aprimary alkylamine.CH 2 CH 2 BrNaN 3CH 2 CH 2 N 3Synthesis of <strong>Amines</strong> via AzidesS N 2 reaction, followed by reduction, gives aprimary alkylamine.CH 2 CH 2 BrNaN 3CH 2 CH 2 N 3CH 2 CH 2 NH 2(89%)(74%)1. LiAlH 42. H 2 Oazides may also bereduced by catalytichydrogenationCH 2 CH 2 NH 2(89%)(74%)1. LiAlH 42. H 2 OSynthesis of <strong>Amines</strong> via NitrilesS N 2 reaction, followed by reduction, gives aprimary alkylamine.CH 3 CH 2 CH 2 CH 2 BrNaCNCH 3 CH 2 CH 2 CH 2 CNS N 2 reaction, followed by reduction, gives aprimary alkylamine.CH 3 CH 2 CH 2 CH 2 BrSynthesis of <strong>Amines</strong> via NitrilesNaCNCH 3 CH 2 CH 2 CH 2 CN(69%)H 2 (100 atm), Ninitriles may also bereduced by lithiumaluminum hydride(69%)H 2 (100 atm), NiCH 3 CH 2 CH 2 CH 2 CH 2 NH 2(56%)CH 3 CH 2 CH 2 CH 2 CH 2 NH 2(56%)