Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
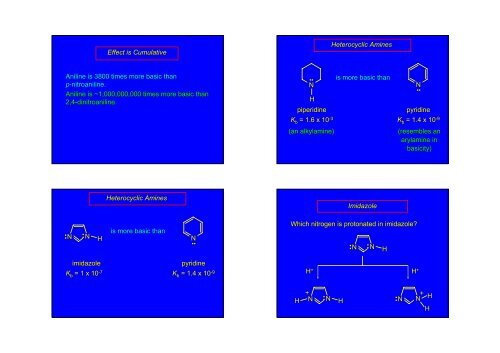
Effect is CumulativeHeterocyclic <strong>Amines</strong>Aniline is 3800 times more basic thanp-nitroaniline.Aniline is ~1,000,000,000 times more basic than2,4-dinitroaniline.••NHpiperidineis more basic thanN ••pyridineK b = 1.6 x 10 -3 K b = 1.4 x 10 -9(an alkylamine)(resembles anarylamine inbasicity)Heterocyclic <strong>Amines</strong>Imidazole••N•• NHis more basic thanN ••Which nitrogen is protonated in imidazole?••N•• NHimidazolepyridineK b = 1 x 10 -7 K b = 1.4 x 10 -9H + H +H+N • N H••N+NHH