Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
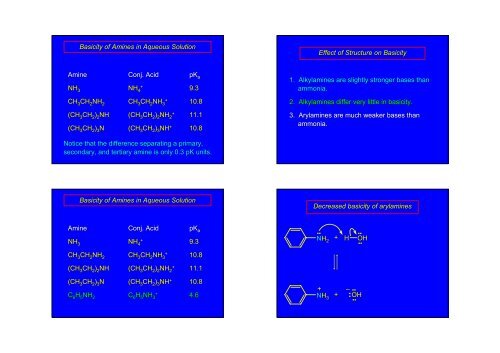
Basicity of <strong>Amines</strong> in Aqueous SolutionEffect of Structure on BasicityAmineNH 3CH 3 CH 2 NH 2(CH 3 CH 2 ) 2 NH(CH 3 CH 2 ) 3 NConj. AcidpK aNH + 4 9.3CH 3 CH 2 NH + 3 10.8(CH 3 CH 2 ) 2 NH + 2 11.1(CH 3 CH 2 ) 3 NH + 10.81. Alkylamines are slightly stronger bases thanammonia.2. Alkylamines differ very little in basicity.3. Arylamines are much weaker bases thanammonia.Notice that the difference separating a primary,secondary, and tertiary amine is only 0.3 pK units.Basicity of <strong>Amines</strong> in Aqueous SolutionDecreased basicity of arylaminesAmineNH 3Conj. AcidpK aNH + 4 9.3••NH 2+ H OH••••CH 3 CH 2 NH 2CH 3 CH 2 NH + 3 10.8(CH 3 CH 2 ) 2 NH(CH 3 CH 2 ) 2 NH + 2 11.1(CH 3 CH 2 ) 3 NC 6 H 5 NH 2(CH 3 CH 2 ) 3 NH + 10.8C 6 H 5 NH + 3 4.6+ –NH 3+ •• OH••••