Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
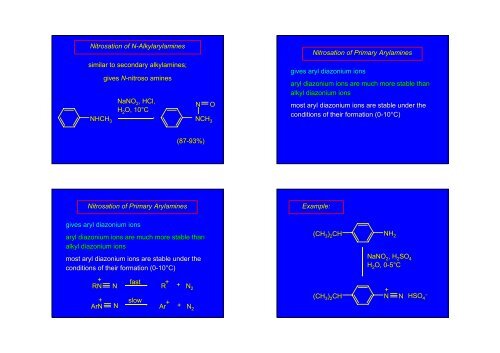
Nitrosation of N-AlkylarylaminesNsimilar to secondary alkylamines;NHCH 3gives N-nitrosoaminesNaNO 2 , HCl,H 2 O, 10°CNONCH 3Nitrosation of Primary Arylaminesgives aryl diazonium ionsaryl diazonium ions are much more stable thanalkyl diazonium ionsmost aryl diazonium ions are stable under theconditions of their formation (0-1010°C)(87-93%)Nitrosation of Primary ArylaminesExample:gives aryl diazonium ionsaryl diazonium ions are much more stable thanalkyl diazonium ionsmost aryl diazonium ions are stable under theconditions of their formation (0-1010°C)(CH 3 ) 2 CHNH 2NaNO 2 , H 2 SO 4H 2 O, 0-5°C0+ fastRNN+ArNNslowR + + N 2Ar + + N 2+(CH 3 ) 2 CH N NHSO – 4