Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
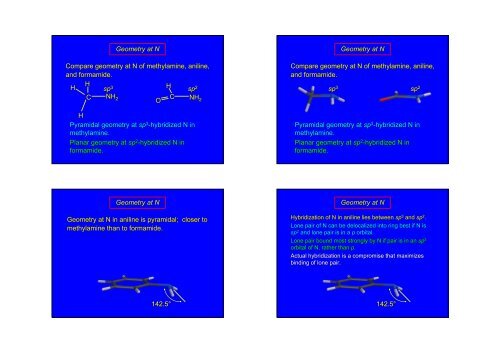
Geometry at NCompare geometry at N of methylamine, aniline,and formamide.HH sp 3Hsp 2C NH 2CONH 2HPyramidal geometry at sp 3 -hybridized N inmethylamine.Planar geometry at sp 2 -hybridized N informamide.Compare geometry at N of methylamine, aniline,and formamide.sp 3Geometry at Nsp 2Pyramidal geometry at sp 3 -hybridized N inmethylamine.Planar geometry at sp 2 -hybridized N informamide.Geometry at NGeometry at N in aniline is pyramidal; closer tomethylamine than to formamide.Geometry at NHybridization of N in aniline lies between sp 3 and sp 2 .Lone pair of N can be delocalized into ring best if N issp 2 and lone pair is in a p orbital.Lone pair bound most strongly by N if pair is in an sp 3orbital of N, rather than p.Actual hybridization is a compromise that maximizesbinding of lone pair.142.5°142.5°