Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
Ch18 Amines(pdf) - KFUPM Open Courseware
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
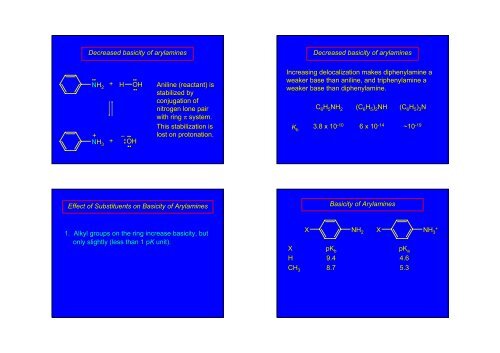
Decreased basicity of arylaminesDecreased basicity of arylamines••NH 2+ H OH••••+ –NH 3+ •• OH••••Aniline (reactant) isstabilized byconjugation ofnitrogen lone pairwith ring π system.This stabilization islost on protonation.Increasing delocalization makes diphenylamine aweaker base than aniline, and triphenylamine aweaker base than diphenylamine.C 6 H 5 NH 2 (C 6 H 5 ) 2 NH (C 6 H 5 ) 3 NK b3.8 x 10 -106 x 10 -14~10~10 -19Effect of Substituents on Basicity of ArylaminesBasicity of Arylamines1. Alkyl groups on the ring increase basicity, , butonly slightly (less than 1 pK unit).XNH 2 X NH + 3X pK bpK aH 9.4 4.6CH 3 8.7 5.3