1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
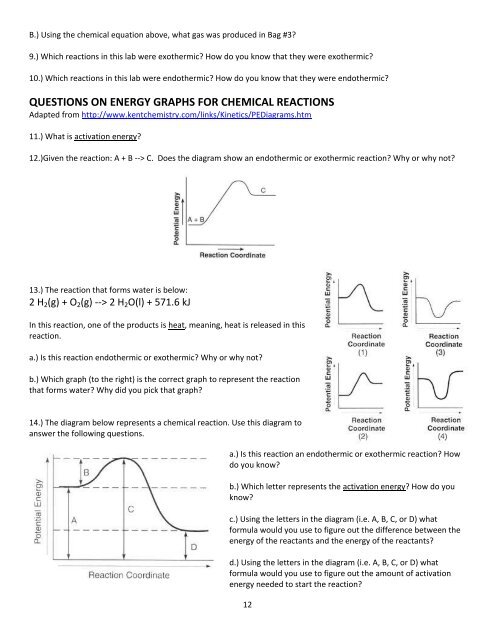
B.) Using the chemical equation above, what gas was produced in Bag #3?9.) Which reactions in <strong>this</strong> lab were exothermic? How do <strong>you</strong> know that they were exothermic?10.) Which reactions in <strong>this</strong> lab were endothermic? How do <strong>you</strong> know that they were endothermic?QUESTIONS ON ENERGY GRAPHS FOR CHEMICAL REACTIONSAdapted from http://www.kentchemistry.com/links/Kinetics/PEDiagrams.htm11.) What is activation energy?12.)Given the reaction: A + B --> C. Does the diagram show an endothermic or exothermic reaction? Why or why not?13.) The reaction that forms water is <strong>be</strong>low:2 H 2 (g) + O 2 (g) --> 2 H 2 O(l) + 571.6 kJ<strong>In</strong> <strong>this</strong> reaction, one of the products is heat, meaning, heat is released in <strong>this</strong>reaction.a.) Is <strong>this</strong> reaction endothermic or exothermic? Why or why not?b.) Which graph (to the right) is the correct graph to represent the reactionthat forms water? Why did <strong>you</strong> pick that graph?14.) The diagram <strong>be</strong>low represents a chemical reaction. Use <strong>this</strong> diagram toanswer the following questions.a.) Is <strong>this</strong> reaction an endothermic or exothermic reaction? Howdo <strong>you</strong> know?b.) Which letter represents the activation energy? How do <strong>you</strong>know?c.) Using the letters in the diagram (i.e. A, B, C, or D) whatformula would <strong>you</strong> use to figure out the difference <strong>be</strong>tween theenergy of the reactants and the energy of the reactants?d.) Using the letters in the diagram (i.e. A, B, C, or D) whatformula would <strong>you</strong> use to figure out the amount of activationenergy needed to start the reaction?12