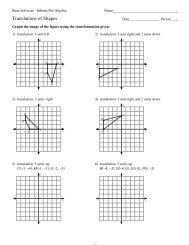
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
carbonate solution. Wait until the bubbling subsides <strong>be</strong>fore adding more acetic acid. When all the acetic acid has <strong>be</strong>enadded, stir for two minutes <strong>be</strong>fore moving on to step 6.7.) When the solution is calm again (there may <strong>be</strong> a few bubbles rising from the bottom of the flask – <strong>this</strong> is normal),move the flask to a hot plate and heat it to boiling. Be careful that the flask does not boil over <strong>be</strong>cause <strong>this</strong> <strong>will</strong> causeerrors in <strong>you</strong>r calculations. Put the flask in the hood.8.) When all of the liquid in the solution has boiled away, remove the flask from the hot plate. The power that <strong>you</strong>observe inside is the product of the reaction, sodium acetate. Once the flask has had a few minutes to cool down toroom temperature, measure and record its weight.Weight of the flask, after the reaction: _______________9.) When done, rinse out the flask and any other glassware <strong>you</strong> used. All waste can go down the sink.Data and ObservationsBefore the chemical reaction1. The chemical equation of the reaction <strong>be</strong>tween baking soda and vinegar isNaHCO 3 (aq) + CH 3 COOH (aq) ----> CO 2 (g) + H 2 O (l) + CH 3 COONa (aq)Show <strong>you</strong>r calculations for how <strong>you</strong> found out how many grams of baking soda to use in <strong>this</strong> lab.Show <strong>you</strong>r calculations for how <strong>you</strong> found out how many grams of sodium acetate to expect in <strong>this</strong> lab.Show <strong>you</strong>r calculations for how <strong>you</strong> found out how many liters of CO 2 to expect from <strong>this</strong> lab.2. Write down 3 physical properties of baking soda.3. Write down 3 physical properties of vinegar.54