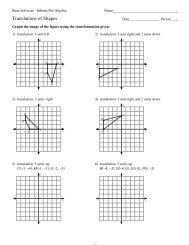
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
4. What was the mass of the empty 500 mL flask? _________________After the Chemical Reaction5. Write down 3 observations about the chemical reaction after <strong>you</strong> mixed the baking soda and vinegar.6. What was the weight of the 500-mL flask after the reaction? ______________7. How many grams of sodium acetate were produced? Show <strong>you</strong>r calculations for how <strong>you</strong> got <strong>you</strong>r answer.8. Using the weight of sodium acetate that <strong>you</strong> got and the weight of sodium acetate that <strong>you</strong> were supposed to get,calculate the percent yield for <strong>this</strong> experiment. Show <strong>you</strong>r calculations <strong>be</strong>low.Results and Discussion1. Why do scientists use stoichiometry? How did it help us in <strong>this</strong> lab? What did we figure out from using it?2. Did a chemical reaction take place? How do <strong>you</strong> know? What evidence tells <strong>you</strong> whether a chemical reactionhappened or not?3. Was <strong>you</strong>r percent yield for sodium acetate 100%? Why or why not?4. Do <strong>you</strong> think it is common for scientists to get a 100% percent yields for chemical reactions? Why or why not?55