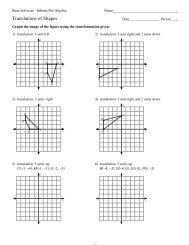
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
10. The unbalanced synthesis reaction <strong>be</strong>tween aluminum metal and oxygen is:Al (s) + O 2 (g) ===> Al 2 O 3 (s).If 6.02 X 10 25 molecules of aluminum oxide are produced, then how many grams of aluminum metal and oxygengas are needed?11. The decomposition of mercury (II) oxide to produce liquid mercury and gaseous oxygen is shown in <strong>this</strong> unbalancedreaction:HgO (s) + heat ===> Hg (l) + O 2 (g)How many grams of mercury (II) oxide are needed to produce 100.3 grams of liquid mercury ? What volume ofoxygen gas is formed at STP?12. The unbalanced synthesis reaction of hydrogen gas with chlorine gas produces hydrogen chloride gas:H 2 (g) + Cl 2 (g) ===> HCl (g)How many grams of hydrogen gas are needed to produce 146.4 grams of hydrogen chloride gas? What volume ofchlorine gas, at STP, is needed?50