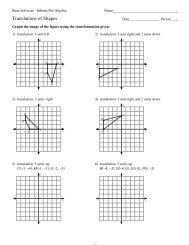
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
4. Determine the num<strong>be</strong>r of moles of carbon dioxide gas, water, and sodium chloride formed by the reaction of 42.0grams of sodium bicarbonate (baking soda/NaHCO 3 ) reacting with excess hydrochloric acid. The reaction is:NaHCO 3 (s) + HCl (aq) ===> CO 2 (g) + H 2 O (l) + NaCl (aq)5. The unbalanced decomposition reaction of butane gas in excess oxygen produces carbon dioxide gas and watervapor: C 4 H 10 (l) + O 2 (g) ===> CO 2 (g) + H 2 O (l). Starting with 11.6 grams of butane, how many grams ofcarbon dioxide gas are formed at STP? What is the volume of the water vapor product?6. The burning of solid sulfur in air produces sulfur dioxide gas.S + O 2 SO 2Balance the reaction. How many moles and molecules (particles) of sulfur dioxide does the burning of 3 moles ofsulfur form? Calculate the volume of sulfur dioxide produced at STP.48