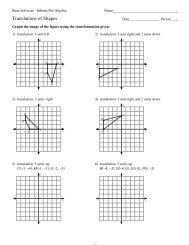
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Example #2The chemical reaction for producing pure copper (refining copper) is <strong>be</strong>low. CuO = copper oxide2 CuO (s) + C(s) -----> 2 Cu(s) + CO 2 (g)___moles ___moles ___moles ___moles1.) Is <strong>this</strong> reaction balanced? How do <strong>you</strong> know?2.) Write down the amount of moles <strong>you</strong> see for each reactant and product under the equation.3.) A refiner needs to convert 955.0 g of CuO to pure Cu. What mass of C is needed for <strong>this</strong> reaction?***Remem<strong>be</strong>r, <strong>you</strong> need to convert to moles in order to use the chemical equation or the recipe!HOMEWORK Complete the problems <strong>be</strong>low on a SEPARATE piece of paper!46