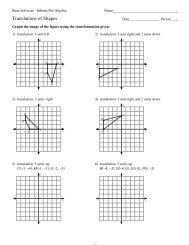
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
6.) If our actual yield was 13 grams of sodium chloride, what would our percent yield for <strong>this</strong> reaction <strong>be</strong>?Formula for percent yield is:Example: I expected to get 30 grams; I ended up getting 25 grams.7.) Is the answer in #6 reasonable? If so, explain why <strong>you</strong> think <strong>this</strong> was a reasonable answer.8.) What are some factors that might cause the percent yield to <strong>be</strong> lower than 100%? Make sure <strong>you</strong> discuss specificexamples of how <strong>this</strong> might happen.<strong>In</strong>troduction<strong>In</strong> <strong>this</strong> lab, <strong>you</strong> <strong>will</strong> need to do a reaction where baking soda <strong>will</strong> react with an excess of vinegar. By doing <strong>this</strong>, <strong>you</strong> <strong>will</strong>(hopefully!) ensure that <strong>you</strong> <strong>will</strong> get 100% actual yield for the reaction.For our reaction, we <strong>will</strong> need to use 0.05 moles of baking soda, which we <strong>will</strong> call by its chemical name, sodiumhydrogen carbonate (NaHCO 3 ) for the rest of <strong>this</strong> lab. If we use much more than 0.05 moles of baking soda, the reaction<strong>will</strong> <strong>be</strong> too large and we <strong>will</strong> rest having some of the reaction products pour over the side of the flask when we mix itwith the vinegar (which we call acetic acid – CH 3 COOH).<strong>In</strong> the space <strong>be</strong>low, use dimensional analysis to calculate the amount of sodium hydrogen carbonate we <strong>will</strong> need for<strong>this</strong> lab:FOR THIS LAB, WE WILL USE ______________GRAMS OF SODIUM HYDROGEN CARBONATE52