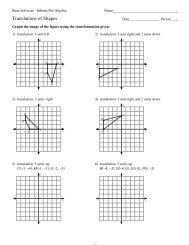
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
1 Name: Date: Period: Project Description: In this project, you will be ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Using stoichiometry, how many moles and grams of sodium acetate do we expect to get? Show <strong>you</strong>r calculations <strong>be</strong>low.Using stoichiometry, how many moles and liters of CO 2 do we expect to get? Show <strong>you</strong>r calculations <strong>be</strong>low.Materials_____grams of baking soda (how much did <strong>you</strong> calculate <strong>you</strong> would need in the introduction?)150 mL of vinegar1 cupA 500 mL flaskStirring rod30 ml of waterHot plateGraduated cylinderMethods<strong>In</strong> <strong>this</strong> section, <strong>you</strong> <strong>will</strong> now carry out the reaction that was discussed at the <strong>be</strong>ginning of <strong>this</strong> lab.1.) Observe 3 physical properties of vinegar and baking soda. Write down <strong>you</strong>r observations in the Data & Observationsection of <strong>this</strong> lab.2.) <strong>In</strong> a cup, measure out the weight of sodium hydrogen carbonate that <strong>you</strong> calculated <strong>you</strong> would need in theintroduction section of <strong>this</strong> lab. Don’t forget to tare (zero) the weight of the cup. Make sure the amount that <strong>you</strong> use isas close as possible to the amount that <strong>you</strong> calculated. Write the exact amount of sodium hydrogen carbonate that <strong>you</strong>used here:Amount of sodium hydrogen carbonate that <strong>you</strong> actually used: _____________________grams3.) Add 30 mL of water to the cup containing the sodium hydrogen carbonate. Stir the solution until most or all of it isdissolved (if a little won’t dissolve, that’s okay).4.) Weigh a 500 mL flask. You <strong>will</strong> need the weight of an empty flask at the end of the lab.Weight of empty 500 mL flask: __________________ grams5.) Add the sodium hydrogen carbonate solution to the empty 500 mL flask.6.) <strong>In</strong> a graduated cylinder, measure out 150 mL of acetic acid (aka vinegar) and slowly add it to the sodium hydrogencarbonate solution. You <strong>will</strong> observe the formation of bubbles when the acetic acid is added to the sodium hydrogen53