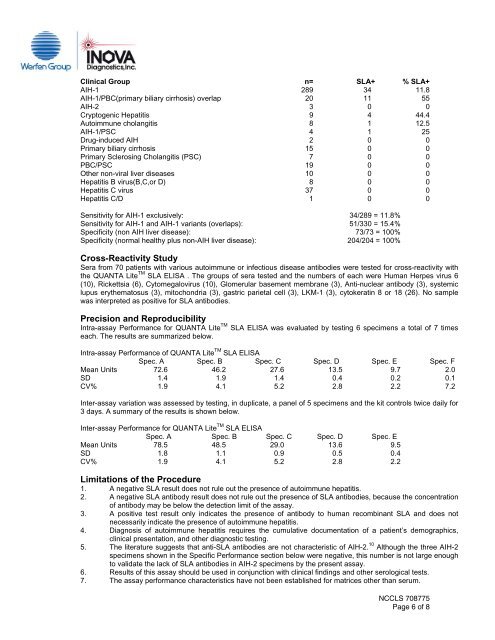
Clinical Group n= <strong>SLA</strong>+ % <strong>SLA</strong>+AIH-1 289 34 11.8AIH-1/PBC(primary biliary cirrhosis) overlap 20 11 55AIH-2 3 0 0Cryptogenic Hepatitis 9 4 44.4Autoimmune cholangitis 8 1 12.5AIH-1/PSC 4 1 25Drug-induced AIH 2 0 0Primary biliary cirrhosis 15 0 0Primary Sclerosing Cholangitis (PSC) 7 0 0PBC/PSC 19 0 0Other non-viral liver diseases 10 0 0Hepatitis B virus(B,C,or D) 8 0 0Hepatitis C virus 37 0 0Hepatitis C/D 1 0 0Sensitivity for AIH-1 exclusively: 34/289 = 11.8%Sensitivity for AIH-1 and AIH-1 variants (overlaps): 51/330 = 15.4%Specificity (non AIH liver disease): 73/73 = 100%Specificity (normal healthy plus non-AIH liver disease): 204/204 = 100%Cross-Reactivity StudySera from 70 patients with various autoimmune or infectious disease antibodies were tested for cross-reactivity withthe <strong>QUANTA</strong> <strong>Lite</strong> TM <strong>SLA</strong> ELISA . The groups of sera tested and the numbers of each were Human Herpes virus 6(10), Rickettsia (6), Cytomegalovirus (10), Glomerular basement membrane (3), Anti-nuclear antibody (3), systemiclupus erythematosus (3), mitochondria (3), gastric parietal cell (3), LKM-1 (3), cytokeratin 8 or 18 (26). No samplewas interpreted as positive for <strong>SLA</strong> antibodies.Precision and ReproducibilityIntra-assay Performance for <strong>QUANTA</strong> <strong>Lite</strong> TM <strong>SLA</strong> ELISA was evaluated by testing 6 specimens a total of 7 timeseach. The results are summarized below.Intra-assay Performance of <strong>QUANTA</strong> <strong>Lite</strong> TM <strong>SLA</strong> ELISASpec. A Spec. B Spec. C Spec. D Spec. E Spec. FMean Units 72.6 46.2 27.6 13.5 9.7 2.0SD 1.4 1.9 1.4 0.4 0.2 0.1CV% 1.9 4.1 5.2 2.8 2.2 7.2Inter-assay variation was assessed by testing, in duplicate, a panel of 5 specimens and the kit controls twice daily for3 days. A summary of the results is shown below.Inter-assay Performance for <strong>QUANTA</strong> <strong>Lite</strong> TM <strong>SLA</strong> ELISASpec. A Spec. B Spec. C Spec. D Spec. EMean Units 78.5 48.5 29.0 13.6 9.5SD 1.8 1.1 0.9 0.5 0.4CV% 1.9 4.1 5.2 2.8 2.2Limitations of the Procedure1. A negative <strong>SLA</strong> result does not rule out the presence of autoimmune hepatitis.2. A negative <strong>SLA</strong> antibody result does not rule out the presence of <strong>SLA</strong> antibodies, because the concentrationof antibody may be below the detection limit of the assay.3. A positive test result only indicates the presence of antibody to human recombinant <strong>SLA</strong> and does notnecessarily indicate the presence of autoimmune hepatitis.4. Diagnosis of autoimmune hepatitis requires the cumulative documentation of a patient’s demographics,clinical presentation, and other diagnostic testing.5. The literature suggests that anti-<strong>SLA</strong> antibodies are not characteristic of AIH-2. 10 Although the three AIH-2specimens shown in the Specific Performance section below were negative, this number is not large enoughto validate the lack of <strong>SLA</strong> antibodies in AIH-2 specimens by the present assay.6. Results of this assay should be used in conjunction with clinical findings and other serological tests.7. The assay performance characteristics have not been established for matrices other than serum.NCCLS <strong>708775</strong>Page 6 of 8
References1. Krawitt EL. Autoimmune hepatitis. N Engl J Med. 1996;334(14):897-903.2. Krawitt EL. Can you recognize autoimmune hepatitis? Postgrad Med. 1998;104(2):145-149, 152.3. Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteriafor diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929-938.4. Obermayer-Straub P, Strassburg CP, Manns MP. Autoimmune hepatitis. J Hepatol. 2000;32(1 Suppl):181-197.5. Manns MP, Strassburg CP. Autoimmune hepatitis: clinical challenges. Gastroenterology. 2001;120(6):1502-1517.6. Czaja AJ, Homburger HA. Autoantibodies in liver disease. Gastroenterology. 2001;120(1):239-249.7. Meyer zum Buschenfelde KH, Lohse AW. Autoimmune hepatitis. N Engl J Med. 1995 Oct 12;333(15):1004-1005.8. Kanzler S, Weidemann C, Gerken G, Lohr HF, Galle PR, Meyer zum Buschenfelde KH, Lohse AW. Clinicalsignificance of autoantibodies to soluble liver antigen in autoimmune hepatitis. J Hepatol. 1999;31(4):635-640.9. McFarlane IG. The relationship between autoimmune markers and different clinical syndromes inautoimmune hepatitis. Gut. 1998;42:599-602.10. Ballot E, Homberg JC, Johanet C. Antibodies to soluble liver antigen: an additional marker in type 1 autoimmunehepatitis. J Hepatol. 2000;33(2):208-215.11. Lapierre P, Hajoui O, Homberg JC, Alvarez F. Formiminotransferase cyclodeaminase is an organ-specificautoantigen recognized by sera of patients with autoimmune hepatitis. Gastroenterology. 1999Mar;116(3):643-649.12. Manns M, Gerken G, Kyriatsoulis A, Staritz M, Meyer zum Buschenfelde KH. Characterization of a newsubgroup of autoimmune chronic active hepatitis by autoantibodies against a soluble liver antigen. Lancet.1987;1:292-294.13. Stechemesser E, Klein R, Berg PA. Characterization and clinical relevance of liver-pancreas antibodies inautoimmune hepatitis. Hepatology. 1993;18(1):1-9.14. Wies I, Brunner S, Henninger J, Herkel J, Kanzler S, Meyer zum Buschenfelde KH, Lohse AW. Identificationof target antigen for <strong>SLA</strong>/LP autoantibodies in autoimmune hepatitis. Lancet. 2000 29;355(9214):1510-1515.15. Costa M, Rodriguez-Sanchez JL, Czaja AJ, Gelpi C. Isolation and characterization of cDNA encoding theantigenic protein of the human tRNP(Ser)Sec complex recognized by autoantibodies from patients withtype-1 autoimmune hepatitis. Clin Exp Immunol. 2000;121(2):364-374.16. Wachter B, Kyriatsoulis A, Lohse AW, Gerken G, Meyer zum Buschenfelde KH, Manns MP.Characterization of liver cytokeratin as a major target antigen of anti-<strong>SLA</strong> antibodies. J Hepatol.1990;11(2):232-239.17. Wesierska-Gadek J, Grimm R, Hitchman E, Penner E. Members of the glutathione S-transferase genefamily are antigens in autoimmune hepatitis. Gastroenterology. 1998 Feb;114(2):329-335.18. Klein R, Berg PA. Glutathione S-transferase as a major autoantigen in anti-<strong>SLA</strong>-positive autoimmunehepatitis. Gastroenterology. 1998;116(4):1015-1016.19. Manns MP. Antibodies to soluble liver antigen: specific marker of autoimmune hepatitis. J Hepatol.2000;33(2):326-328.20. Czaja AJ, Carpenter HA, Manns MP. Antibodies to soluble liver antigen, P450IID6, and mitochondrialcomplexes in chronic hepatitis. Gastroenterology. 1993;105(5):1522-1528.21. Dalekos GN, Wedemeyer H, Obermayer-Straub P, Kayser A, Barut A, Frank H, Manns MP. Epitopemapping of cytochrome P4502D6 autoantigen in patients with chronic hepatitis C during alpha-interferontreatment. J. Hepatol. 1999;30(3):366-375.22. Czaja AJ, Donaldson PT, Lohse AW. Antibodies to soluble liver antigen/liver pancreas and HLA risk factorsfor type 1 autoimmune hepatitis. Am J Gastroenterol. 2002;97:413-419.23. Biosafety in Microbiological and Biomedical Laboratories. Center for Disease Control/National Institute ofHealth, 1999, Fourth Edition, (HHS Pub. # (CDC) 93-8395).24. National Committee for Clinical Laboratory Standards. 1991. Internal quality control: Principles anddefinitions; Approved Guideline, NCCLS Document C24-A, Vol.11(6).25. Nishioka M, Morshed SA, Parveen S, Kono K, Matsuoka H, Manns MP. Antibodies to P450IID6, <strong>SLA</strong>, PDH-E2 and BCKD-E2 in Japanese patients with chronic hepatitis. J Gastroenterol Hepatol. 1997;12(12):862-868.NCCLS <strong>708775</strong>Page 7 of 8