Argonaut Quest Training Workshop 2 (pdf) - Artisan Scientific
Argonaut Quest Training Workshop 2 (pdf) - Artisan Scientific
Argonaut Quest Training Workshop 2 (pdf) - Artisan Scientific
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
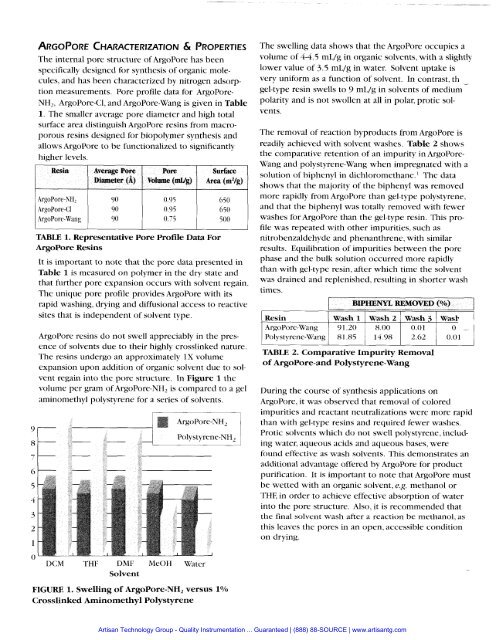
The internal pore structure of ArgoPore has beenspecifically designed for synthesis of organic molecules,and has been characterized by nitrogen adsorptionmeasurements. Pore profile data for ArgoPore-NH,, ArgoPore-CI, and ArgoPore-Wang is given in Table1. The smaller average pore diameter and high totalsurface area distinguish ArgoPore resins from macroporousresins designed for biopolymer synthesis andallows At-goPore to be functionalized to significantlyhigher levels.ResinAverage PureDiameter (A)PoreVolume (Wg)SurfaceArea (m2/g)I 1 I I ITABLE 1. Representative Pore Profile Data ForArgoPore ResinsIt is important to note that the pore data presented inTable 1 is measured on polymer in the dry state andthat further pore expansion occurs with solvent regain.The unique pore profile provides ArgoPore with itsrapid washing, drying and diffusional access to reactivesites that is independent of solvent type.ArgoPore resins do not swell appreciably in the presenceof solvents due to their highly crosslinked nature.The resins undergo an approximately 1X volumeexpansion upon addition of organic solvent due to solventregain into the pore structure. In Figure 1 thevolume per gram of ArgoPore-NH, is compared to a gelaminomethyl polystyrene for a series of solvents.The swelling data shows that the ArgoPore occupies avolume of 4-4.5 mL/g in organic solvents, with a slightlylower value of 3.5 mL/g in water. Solvent uptake isvery uniform as a function of solvent. In contrast, th -gel-type resin swells to 9 mL/g in solvents of mediumpolarity and is not swollen at all in polar, protic solvents.The removal of reaction byproducts from ArgoPore isreadily achieved with solvent washes. Table 2 showsthe comparative retention of an impurity in ArgoPore-Wang and polystyrene-Wang when impregnated with asolution of biphenyl in dichloromethane.' The datashows that the majority of the biphenyl was removedmore rapidly from ArgoPore than gel-type polystyrene,and that the biphenyl was totally removed with fewerwashes for ArgoPore than the gel-type resin. This profilewas repeated with other impurities, such asnitrobenzaldehyde and phenanthrene, with similarresults. Equilibration of impurities between the porephase and the bulk solution occurred more rapidlythan with gel-type resin, after which time the solventwas drained and replenished, resulting in shorter washtimes.BIPHENYL REMOVED (O/o)1I- I I I,ResinWash 1 Wash 2 Wash 3 Wash 1ArgoPore-Wang 91.208.00 0.01 0 -Polystyrene-Wang 81.85 14 98 2.62 0.01TABLE 2. Comparative Impurity Removalof ArgoPore-and Polystyrene-WangDuring the course of synthesis applications onArgoPore, it was observed that removal of coloredimpurities and reactant neutralizations were more rapidthan with gel-type resins and required fewer washes.Protic solvents which do not swell polystyrene, includingwater, aqueous acids and aqueous bases, werefound effective as wash solvents. This demonstrates anadditional advantage offered by ArgoPore for productpurification. It is important to note that ArgoPore mustbe wetted with an organic solvent, e.g. methanol orTHE in order to achieve effective absorption of waterinto the pore structure. Also, it is recommended thatthe final solvent wash after a reaction be methanol, asthis leaves the pores in an open, accessible conditionon drying.DCM THF DMF MeOH WaterSolventFIGURE 1. Swelling of ArgoPore-NH, versus 1%Crosslinked Aminomethyl Polystyrene<strong>Artisan</strong> Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | www.artisantg.com