Argonaut Quest Training Workshop 2 (pdf) - Artisan Scientific
Argonaut Quest Training Workshop 2 (pdf) - Artisan Scientific
Argonaut Quest Training Workshop 2 (pdf) - Artisan Scientific
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
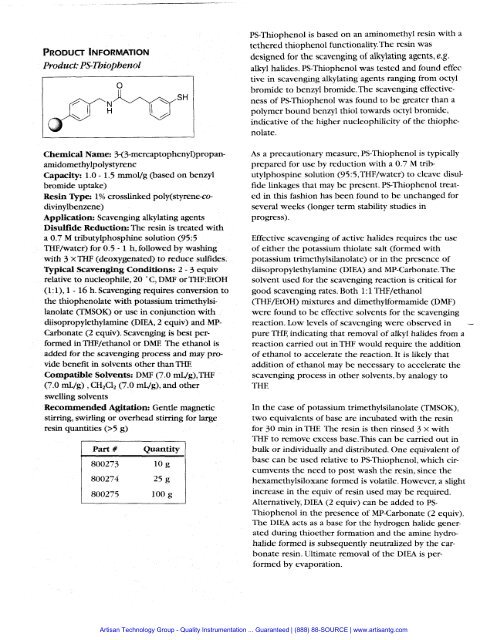
PRODUCT INFORMATIONProduct: PS-ThiophenolChemical Name: 3-(3-mercaptopheny1)propanamidomethylpolystyreneCapacity: 1.0 - 1.5 mmol/g (based on benzylbromide uptake)Resin Type: 1% crosslinked poly(styrene-codivinylbenzene)Application: Scavenging alkylating agentsDisulfide Reduction: The resin is treated witha 0.7 M tributylphosphine solution (95:5THF/water) for 0.5 - 1 h, followed by washingwith 3 xTI3.F (deoxygenated) to reduce sulfides.Typical Scavenging Conditions: 2 - 3 equivrelative to nucleophile, 20 " C, DMF or THF:EtOH(1:1), 1 - 16 h. Scavenging requires conversion tothe thiophenolate with potassium trimethylsilanolate(TMSOK) or use in conjunction withdiisopropylethylamine (DIEA, 2 equiv) and MP-Carbonate (2 equiv). Scavenging is best performedinTHJ?/ethanol or DME The ethanol isadded for the scavenging process and may providebenefit in solvents other than 'ITECompatible Solvents. DMF (7.0 mug), THF(7.0 rnL/g) , CH,C12 (7.0 mL/g), and otherswelling solventsRecommended Agitation: Gentle magneticstirring, swirling or overhead stirring for largeresin quantities (>5 g)Part #QuantityPS-Thiophenol is based on an aminomethyl resin with atethered thiophenol functionality.The resin wasdesigned for the scavenging of alkylating agents, e.g.alkyl halides. PS-Thiophenol was tested and found effec.tive in scavenging alkylating agents ranging from octylbromide to benzyl brornide.The scavenging effectivenessof PS-Thiophenol was found to be greater than apolymer bound benzyl thiol towards octyl bromide,indicative of the higher nucleophilicity of the thiophenolate.As a precautionary measure, PS-Thiophenol is typicallyprepared for use by reduction with a 0.7 M tributylphospinesolution (95:5,THF/water) to cleave disulfidelinkages that may be present. PS-Thiophenol treatedin this fashion has been found to be unchanged forseveral weeks (longer term stability studies inprogress).Effective scavenging of active halides requires the useof either the potassium thiolate salt (formed withpotassium trimethylsilanolate) or in the presence ofdiisopropylethylamine (DIEA) and MP-CarbonateThesolvent used for the scavenging reaction is critical forgood scavenging rates. Both 1:1 THF/ethanol(THF/EtOH) mixtures and dimethylformamide (Dm)were found to be effective solvents for the scavengingreaction. Low levels of scavenging were observed in -pure THE indicating that removal of alkyl halides from areaction carried out in THF would require the additionof ethanol to accelerate the reaction. It is likely thataddition of ethanol may be necessary to accelerate thescavenging process in other solvents, by analogy toTHEIn the case of potassium trimethylsilanolate (TMSOK),two equivalents of base are incubated with the resinfor 30 min inTHE The resin is then rinsed 3 x withTHF to remove excess base.This can be carried out inbulk or individually and distributed. One equivalent ofbase can be used relative to PS-Thiophenol, which circumventsthe need to post wash the resin, since thehexamethylsiloxane formed is volatile. However, a slightincrease in the equiv of resin used may be required.Alternatively, DIEA (2 equiv) can be added to PS-Thiophenol in the presence of MP-Carbonate (2 equiv).The DIEA acts as a base for the hydrogen halide generatedduring thioether formation and the amine hydrohalideformed is subsequently neutralized by the carbonateresin. Ultimate removal of the DIM is performedby evaporation.<strong>Artisan</strong> Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | www.artisantg.com