Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
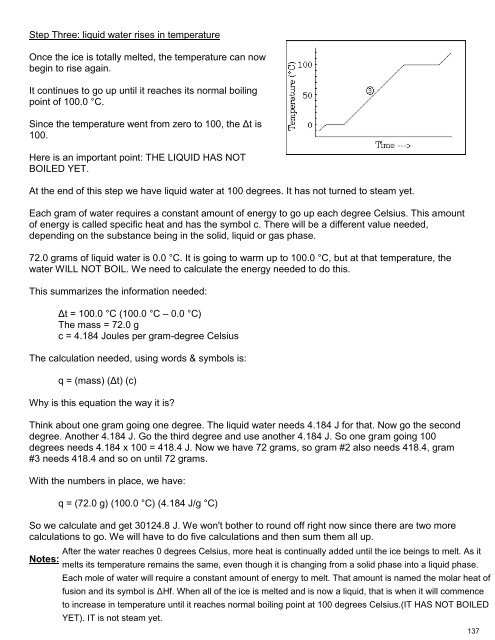
Step Three: liquid water rises in temperature<br />
Once the ice is totally melted, the temperature can now<br />
begin to rise again.<br />
It continues to go up until it reaches its normal boiling<br />
point of 100.0 °C.<br />
Since the temperature went from zero to 100, the Δt is<br />
100.<br />
Here is an important point: THE LIQUID HAS NOT<br />
BOILED YET.<br />
At the end of this step we have liquid water at 100 degrees. It has not turned to steam yet.<br />
Each gram of water requires a constant amount of energy to go up each degree Celsius. This amount<br />
of energy is called specific heat and has the symbol c. There will be a different value needed,<br />
depending on the substance being in the solid, liquid or gas phase.<br />
72.0 grams of liquid water is 0.0 °C. It is going to warm up to 100.0 °C, but at that temperature, the<br />
water WILL NOT BOIL. We need to calculate the energy needed to do this.<br />
This summarizes the information needed:<br />
Δt = 100.0 °C (100.0 °C – 0.0 °C)<br />
The mass = 72.0 g<br />
c = 4.184 Joules per gram-degree Celsius<br />
The calculation needed, using words & symbols is:<br />
q = (mass) (Δt) (c)<br />
Why is this equation the way it is?<br />
Think about one gram going one degree. The liquid water needs 4.184 J for that. Now go the second<br />
degree. Another 4.184 J. Go the third degree and use another 4.184 J. So one gram going 100<br />
degrees needs 4.184 x 100 = 418.4 J. Now we have 72 grams, so gram #2 also needs 418.4, gram<br />
#3 needs 418.4 and so on until 72 grams.<br />
With the numbers in place, we have:<br />
q = (72.0 g) (100.0 °C) (4.184 J/g °C)<br />
So we calculate and get 30124.8 J. We won't bother to round off right now since there are two more<br />
calculations to go. We will have to do five calculations and then sum them all up.<br />
Notes: