You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
A increasing the equilibrium constant in favor of products.<br />
B lowering the activation energy required for the reaction to occur.<br />
C raising the temperature at which the reaction occurs.<br />
D increasing the pressure of reactants, thus favoring products.<br />
<strong>Chem</strong>ical Concepts:<br />
The chemical concepts associated with this question are the “catalyst” and “activation energy”.<br />
Activation energy is defined as the minimum amount of energy a reactant needs to obtain in order for<br />
the reaction to take place. This factor is directly affected by a catalyst which is a substance that is<br />
used to lower the activation rate, but is not consumed or used up, in the process. When the energy<br />
required lessens the reaction takes place at a quicker rate, because the time needed to obtain the<br />
necessary amount of energy, is less as the quota is met at a much lower standard.<br />
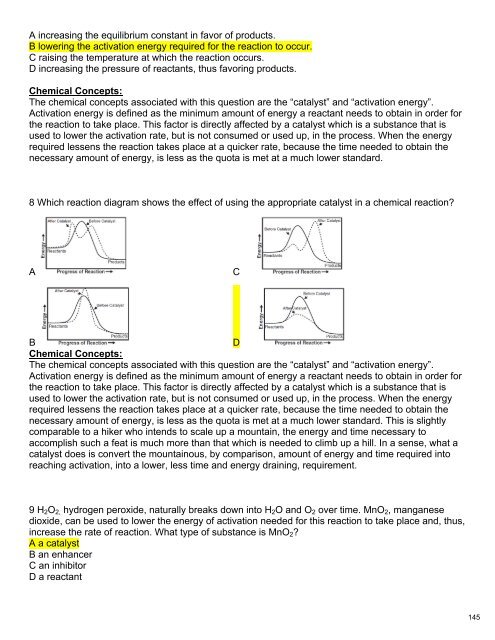
8 Which reaction diagram shows the effect of using the appropriate catalyst in a chemical reaction?<br />
A<br />
C<br />
B<br />
D<br />
<strong>Chem</strong>ical Concepts:<br />
The chemical concepts associated with this question are the “catalyst” and “activation energy”.<br />
Activation energy is defined as the minimum amount of energy a reactant needs to obtain in order for<br />
the reaction to take place. This factor is directly affected by a catalyst which is a substance that is<br />
used to lower the activation rate, but is not consumed or used up, in the process. When the energy<br />
required lessens the reaction takes place at a quicker rate, because the time needed to obtain the<br />
necessary amount of energy, is less as the quota is met at a much lower standard. This is slightly<br />
comparable to a hiker who intends to scale up a mountain, the energy and time necessary to<br />
accomplish such a feat is much more than that which is needed to climb up a hill. In a sense, what a<br />
catalyst does is convert the mountainous, by comparison, amount of energy and time required into<br />
reaching activation, into a lower, less time and energy draining, requirement.<br />
9 H 2 O 2, hydrogen peroxide, naturally breaks down into H 2 O and O 2 over time. MnO 2 , manganese<br />
dioxide, can be used to lower the energy of activation needed for this reaction to take place and, thus,<br />
increase the rate of reaction. What type of substance is MnO 2 ?<br />
A a catalyst<br />
B an enhancer<br />
C an inhibitor<br />
D a reactant