You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
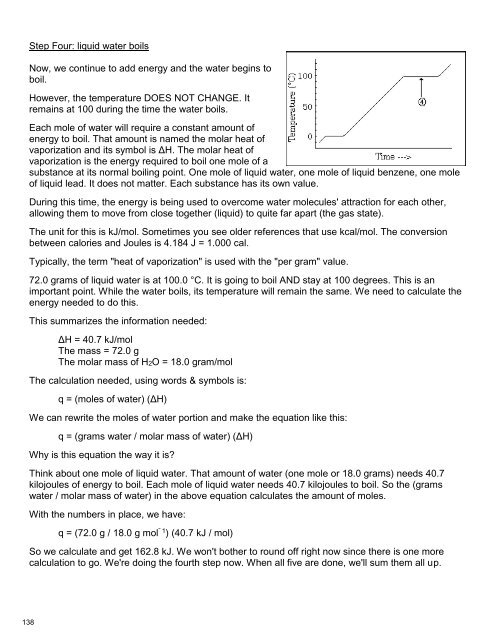
Step Four: liquid water boils<br />
Now, we continue to add energy and the water begins to<br />
boil.<br />
However, the temperature DOES NOT CHANGE. It<br />
remains at 100 during the time the water boils.<br />
Each mole of water will require a constant amount of<br />
energy to boil. That amount is named the molar heat of<br />
vaporization and its symbol is ΔH. The molar heat of<br />
vaporization is the energy required to boil one mole of a<br />
substance at its normal boiling point. One mole of liquid water, one mole of liquid benzene, one mole<br />
of liquid lead. It does not matter. Each substance has its own value.<br />
During this time, the energy is being used to overcome water molecules' attraction for each other,<br />
allowing them to move from close together (liquid) to quite far apart (the gas state).<br />
The unit for this is kJ/mol. Sometimes you see older references that use kcal/mol. The conversion<br />
between calories and Joules is 4.184 J = 1.000 cal.<br />
Typically, the term "heat of vaporization" is used with the "per gram" value.<br />
72.0 grams of liquid water is at 100.0 °C. It is going to boil AND stay at 100 degrees. This is an<br />
important point. While the water boils, its temperature will remain the same. We need to calculate the<br />
energy needed to do this.<br />
This summarizes the information needed:<br />
ΔH = 40.7 kJ/mol<br />
The mass = 72.0 g<br />
The molar mass of H2O = 18.0 gram/mol<br />
The calculation needed, using words & symbols is:<br />
q = (moles of water) (ΔH)<br />
We can rewrite the moles of water portion and make the equation like this:<br />
q = (grams water / molar mass of water) (ΔH)<br />
Why is this equation the way it is?<br />
Think about one mole of liquid water. That amount of water (one mole or 18.0 grams) needs 40.7<br />
kilojoules of energy to boil. Each mole of liquid water needs 40.7 kilojoules to boil. So the (grams<br />
water / molar mass of water) in the above equation calculates the amount of moles.<br />
With the numbers in place, we have:<br />
q = (72.0 g / 18.0 g mol¯1 ) (40.7 kJ / mol)<br />
So we calculate and get 162.8 kJ. We won't bother to round off right now since there is one more<br />
calculation to go. We're doing the fourth step now. When all five are done, we'll sum them all up.