Matrix metalloproteinases (MMPs): Chemical–biological functions ...
Matrix metalloproteinases (MMPs): Chemical–biological functions ...
Matrix metalloproteinases (MMPs): Chemical–biological functions ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
2240 R. P. Verma, C. Hansch / Bioorg. Med. Chem. 15 (2007) 2223–2268<br />
contacts have been made. The linear ClogP model suggests<br />
that the highly hydrophobic molecules will be more<br />
active. Eq. 2 explains 82.7% of variance in log1/K i.<br />
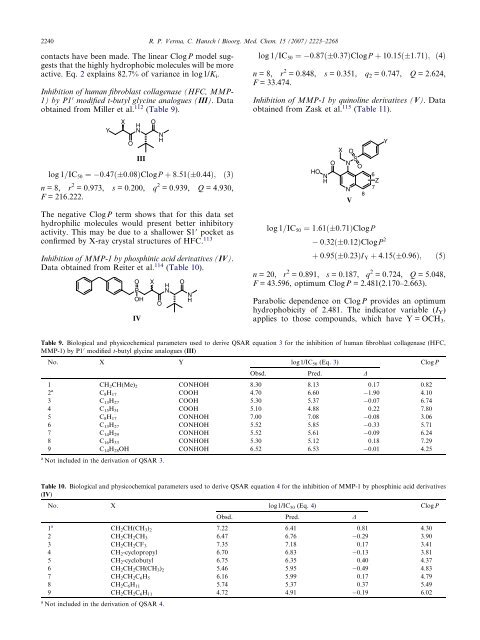
Inhibition of human fibroblast collagenase (HFC, MMP-<br />
1) by P1 0 modified t-butyl glycine analogues (III). Data<br />
obtained from Miller et al. 112 (Table 9).<br />
Y<br />
X<br />
O<br />
H<br />
N<br />
log 1=IC50 ¼ 0:47ð 0:08ÞClogP þ 8:51ð 0:44Þ; ð3Þ<br />
n =8, r 2 = 0.973, s = 0.200, q 2 = 0.939, Q = 4.930,<br />
F = 216.222.<br />
III<br />
The negative ClogP term shows that for this data set<br />
hydrophilic molecules would present better inhibitory<br />
activity. This may be due to a shallower S1 0 pocket as<br />
confirmed by X-ray crystal structures of HFC. 113<br />
Inhibition of MMP-1 by phosphinic acid derivatives (IV).<br />
Data obtained from Reiter et al. 114 (Table 10).<br />
O<br />
P<br />
OH<br />
IV<br />
O<br />
N<br />
H<br />
log 1=IC50 ¼ 0:87ð 0:37ÞClogP þ 10:15ð 1:71Þ; ð4Þ<br />
n =8, r 2 = 0.848, s = 0.351, q2 = 0.747, Q = 2.624,<br />
F = 33.474.<br />
Inhibition of MMP-1 by quinoline derivatives (V). Data<br />
obtained from Zask et al. 115 (Table 11).<br />
HO<br />
N<br />
H<br />
X O<br />
O N<br />
S<br />
O<br />
log 1=IC50 ¼ 1:61ð 0:71ÞClogP<br />
N<br />
V<br />
8<br />
6<br />
Z<br />
7<br />
0:32ð 0:12ÞClogP 2<br />
þ 0:95ð 0:23ÞI Y þ 4:15ð 0:96Þ; ð5Þ<br />
n = 20, r 2 = 0.891, s = 0.187, q 2 = 0.724, Q = 5.048,<br />
F = 43.596, optimum ClogP = 2.481(2.170–2.663).<br />
Parabolic dependence on Clog P provides an optimum<br />
hydrophobicity of 2.481. The indicator variable (IY)<br />
applies to those compounds, which have Y = OCH3.<br />
Table 9. Biological and physicochemical parameters used to derive QSAR equation 3 for the inhibition of human fibroblast collagenase (HFC,<br />
MMP-1) by P10 modified t-butyl glycine analogues (III)<br />
No. X Y log1/IC50 (Eq. 3) ClogP<br />
Obsd. Pred. D<br />
1<br />
2<br />
CH2CH(Me)2 CONHOH 8.30 8.13 0.17 0.82<br />
a<br />
C8H17 COOH 4.70 6.60 1.90 4.10<br />
3 C13H27 COOH 5.30 5.37 0.07 6.74<br />
4 C15H31 COOH 5.10 4.88 0.22 7.80<br />
5 C8H17 CONHOH 7.00 7.08 0.08 3.06<br />
6 C13H27 CONHOH 5.52 5.85 0.33 5.71<br />
7 C14H29 CONHOH 5.52 5.61 0.09 6.24<br />
8 C16H33 CONHOH 5.30 5.12 0.18 7.29<br />
9 C14H28OH CONHOH 6.52 6.53 0.01 4.25<br />
a Not included in the derivation of QSAR 3.<br />
Table 10. Biological and physicochemical parameters used to derive QSAR equation 4 for the inhibition of MMP-1 by phosphinic acid derivatives<br />
(IV)<br />
No. X log1/IC50 (Eq. 4) ClogP<br />
Obsd. Pred. D<br />
1 a<br />
CH2CH(CH3) 2 7.22 6.41 0.81 4.30<br />
2 CH2CH2CH3 6.47 6.76 0.29 3.90<br />
3 CH2CH2CF3 7.35 7.18 0.17 3.41<br />
4 CH2-cyclopropyl 6.70 6.83 0.13 3.81<br />
5 CH2-cyclobutyl 6.75 6.35 0.40 4.37<br />
6 CH2CH2CH(CH3)2 5.46 5.95 0.49 4.83<br />
7 CH2CH2C6H5 6.16 5.99 0.17 4.79<br />
8 CH2C6H11 5.74 5.37 0.37 5.49<br />
9 CH2CH2C6H11 4.72 4.91 0.19 6.02<br />
a Not included in the derivation of QSAR 4.<br />
X<br />
O<br />
H<br />
N<br />
O<br />
N<br />
H<br />
Y