Matrix metalloproteinases (MMPs): Chemical–biological functions ...
Matrix metalloproteinases (MMPs): Chemical–biological functions ...
Matrix metalloproteinases (MMPs): Chemical–biological functions ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2230 R. P. Verma, C. Hansch / Bioorg. Med. Chem. 15 (2007) 2223–2268<br />
S1/2 site<br />
S1/S2 site<br />
HO<br />
HN<br />
X<br />
O<br />
Zn 2+<br />
O<br />
N P<br />
O<br />
OH<br />
N<br />
H<br />
N<br />
P R1 O O<br />
R2 S1' pocket<br />
S2'/S3' site<br />
Figure 7. Structure–activity relationships (SARs) for phosphonamidebased<br />
hydroxamic acids. Reprinted with permission from Ref. 71.<br />
Copyright 2002 American Chemical Society.<br />
The SAR study has shown that the ester group (R1)<br />
seems to have little interaction with the S2 0 /S3 0 site of<br />
<strong>MMPs</strong>, especially of MMP-1. The effects of the substituents<br />
R2 attached to the phosphonamide are important.<br />
Introduction of a long alkoxyalkyl chain at the para<br />
position of the phenyl ring resulted in the significant decrease<br />
of the inhibitory activity against MMP-1, while<br />
the inhibitory activities of other enzymes were maintained<br />
or increased. Thus, the R 2-substituents would<br />
bind to the S1 0 pocket of the MMP enzymes, because<br />
this pocket is deep for most MMP enzymes, but it is<br />
short for MMP-1. On the other hand, bulky substituents<br />
at the para position of the phenyl ring increased the<br />
inhibitory activity for MMP-9. Insertion of an alkyl<br />
chain between the phenyl ring and the phosphonamide<br />
moiety resulted in a slight decrease of the inhibitory<br />
activity for all enzymes, but the alkenyl chain dramatically<br />
decreased the activity for MMP-1. Hydrogen bond<br />
(in case of fluorine atom) or a tight bond of the hydroxa-<br />
H N<br />
Me<br />
peptide backbone<br />
S1' pocket<br />
S2'/3' direction<br />
Figure 6. Binding interaction of N-hydroxy-2(R)-[[(R)-methylphenylphosphinyl]-benzylamino]-4-methylpentanamide<br />
in MMP enzyme.<br />
Reprinted with permission from Ref. 71. Copyright 2002 American<br />
Chemical Society.<br />
P1' substituent: Crucial for potency<br />
and selectivity. Aromatic substituents<br />
are superior, especially R 2 = phenoxy<br />
with the R-configuration at phosphorus.<br />
Zinc-binding group:<br />
A hydroxamic acid is<br />
the superior group<br />
HO<br />
N<br />
H<br />
O<br />
O<br />
R 1<br />
R 2<br />
X<br />
P<br />
N<br />
P1 substituent (only phosphonamides):<br />
Both straight chain and branched alkyl<br />
substituents with R-configuration are tolerated.<br />
Potency for six-memberd ring increases upto<br />
50 times, less for larger rings.<br />
mate to the zinc ion and the p-methoxyphenyl moiety in<br />
the S1 0 pocket have been mentioned to be obligatory.<br />
The oxygen atom of the phosphonamide was positioned<br />
at the hydrogen bond distance with the main chain of<br />
the Leu-164 and Ala-165. Replacement of 1,2,3,4-tetrahydroisoquinoline<br />
ring with other heterocycles provided<br />
insight into the structural requirements of the S1/S2<br />
binding site. Regarding the SAR for the X moiety,<br />
reduction of the ring size resulted in a significant<br />
decrease of the inhibitory activity against all enzymes.<br />
Subsequent modeling work strongly supported the presence<br />
of R-isomer at the phosphorus center. 71<br />
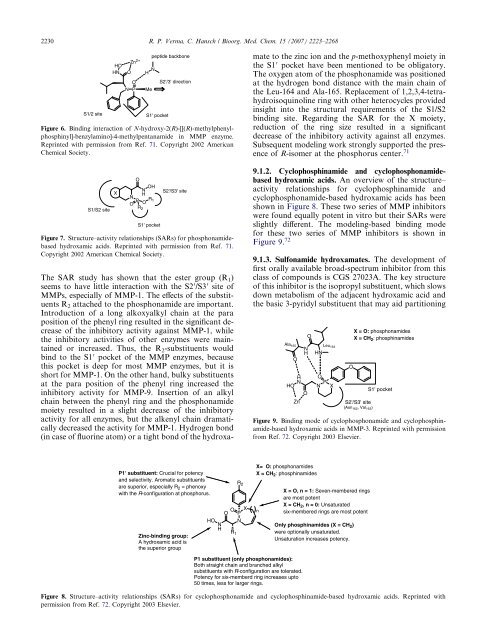
9.1.2. Cyclophosphinamide and cyclophosphonamidebased<br />
hydroxamic acids. An overview of the structure–<br />
activity relationships for cyclophosphinamide and<br />
cyclophosphonamide-based hydroxamic acids has been<br />
shown in Figure 8. These two series of MMP inhibitors<br />
were found equally potent in vitro but their SARs were<br />
slightly different. The modeling-based binding mode<br />
for these two series of MMP inhibitors is shown in<br />
Figure 9. 72<br />
9.1.3. Sulfonamide hydroxamates. The development of<br />
first orally available broad-spectrum inhibitor from this<br />
class of compounds is CGS 27023A. The key structure<br />
of this inhibitor is the isopropyl substituent, which slows<br />
down metabolism of the adjacent hydroxamic acid and<br />
the basic 3-pyridyl substituent that may aid partitioning<br />
X= O: phosphonamides<br />
X = CH 2: phosphinamides<br />
n<br />
X = O, n = 1: Seven-membered rings<br />
are most potent<br />
X = CH 2, n = 0: Unsaturated<br />
six-membered rings are most potent<br />
Only phosphinamides (X = CH 2)<br />
were optionally unsaturated.<br />
Unsaturation increases potency.<br />
Figure 8. Structure–activity relationships (SARs) for cyclophosphonamide and cyclophosphinamide-based hydroxamic acids. Reprinted with<br />
permission from Ref. 72. Copyright 2003 Elsevier.<br />
O<br />
H<br />
N<br />
HO<br />
O<br />
Zn<br />
O<br />
Ala 165 Leu164<br />
N<br />
H<br />
HN<br />
O<br />
P<br />
N X<br />
O<br />
X = O: phosphonamides<br />
X = CH 2: phosphinamides<br />
S2'/S3' site<br />
(Asn 162, Val 163)<br />
S1' pocket<br />
Figure 9. Binding mode of cyclophosphonamide and cyclophosphinamide-based<br />
hydroxamic acids in MMP-3. Reprinted with permission<br />
from Ref. 72. Copyright 2003 Elsevier.