Non-pharmacological interventions for caregivers ... - Update Software
Non-pharmacological interventions for caregivers ... - Update Software
Non-pharmacological interventions for caregivers ... - Update Software
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
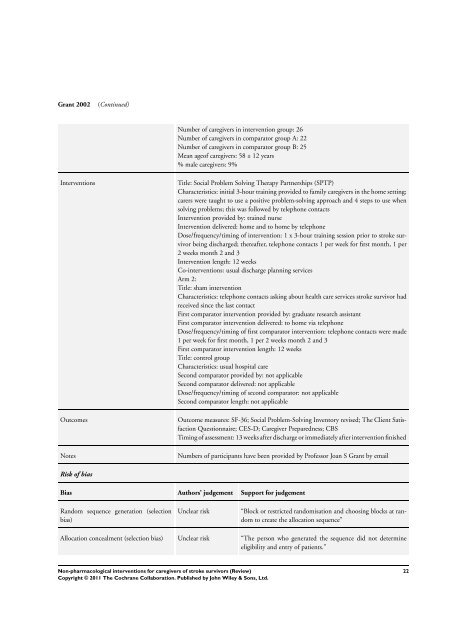
Grant 2002 (Continued)<br />
Number of <strong>caregivers</strong> in intervention group: 26<br />
Number of <strong>caregivers</strong> in comparator group A: 22<br />
Number of <strong>caregivers</strong> in comparator group B: 25<br />
Mean ageof <strong>caregivers</strong>: 58 ± 12 years<br />
% male <strong>caregivers</strong>: 9%<br />
Interventions Title: Social Problem Solving Therapy Partnerships (SPTP)<br />
Characteristics: initial 3-hour training provided to family <strong>caregivers</strong> in the home setting;<br />
carers were taught to use a positive problem-solving approach and 4 steps to use when<br />
solving problems; this was followed by telephone contacts<br />
Intervention provided by: trained nurse<br />
Intervention delivered: home and to home by telephone<br />
Dose/frequency/timing of intervention: 1 x 3-hour training session prior to stroke survivor<br />
being discharged; thereafter, telephone contacts 1 per week <strong>for</strong> first month, 1 per<br />
2 weeks month 2 and 3<br />
Intervention length: 12 weeks<br />
Co-<strong>interventions</strong>: usual discharge planning services<br />
Arm 2:<br />
Title: sham intervention<br />
Characteristics: telephone contacts asking about health care services stroke survivor had<br />
received since the last contact<br />
First comparator intervention provided by: graduate research assistant<br />
First comparator intervention delivered: to home via telephone<br />
Dose/frequency/timing of first comparator intervention: telephone contacts were made<br />
1 per week <strong>for</strong> first month, 1 per 2 weeks month 2 and 3<br />
First comparator intervention length: 12 weeks<br />
Title: control group<br />
Characteristics: usual hospital care<br />
Second comparator provided by: not applicable<br />
Second comparator delivered: not applicable<br />
Dose/frequency/timing of second comparator: not applicable<br />
Second comparator length: not applicable<br />
Outcomes Outcome measures: SF-36; Social Problem-Solving Inventory revised; The Client Satisfaction<br />
Questionnaire; CES-D; Caregiver Preparedness; CBS<br />
Timing of assessment: 13 weeks after discharge or immediately after intervention finished<br />
Notes Numbers of participants have been provided by Professor Joan S Grant by email<br />
Risk of bias<br />
Bias Authors’ judgement Support <strong>for</strong> judgement<br />
Random sequence generation (selection<br />
bias)<br />
Unclear risk “Block or restricted randomisation and choosing blocks at random<br />
to create the allocation sequence”<br />
Allocation concealment (selection bias) Unclear risk “The person who generated the sequence did not determine<br />
eligibility and entry of patients.”<br />
<strong>Non</strong>-<strong>pharmacological</strong> <strong>interventions</strong> <strong>for</strong> <strong>caregivers</strong> of stroke survivors (Review)<br />
Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.<br />
22