Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Photochemistry</strong> <strong>and</strong> <strong>Photophysics</strong> <strong>of</strong> <strong>Coordination</strong> <strong>Compounds</strong>: Copper 89<br />
wards exciplex quenching. It has been hypothesized that emission stems from<br />
the pentacoordinated exciplex itself that deactivates to a pentacoordinated<br />
groundstatespecieswhicheventuallylosesthe“fifth”nucleophiliclig<strong>and</strong>to<br />
regenerate the initial ground state pseudotetrahedral complex [81]. In recent<br />
years it has also been evidenced that some [Cu(NN)2] + complexes exhibit<br />
a prompt luminescence signal with a lifetime <strong>of</strong> the order <strong>of</strong> 13–16 ps, which<br />
is attributed to deactivation <strong>of</strong> 1 MLCT [88], whereas the long-lived component<br />
(above 50 ns) would be phosphorescence from 3 MLCT, which borrows<br />
intensity from upper lying singlet levels [89].<br />
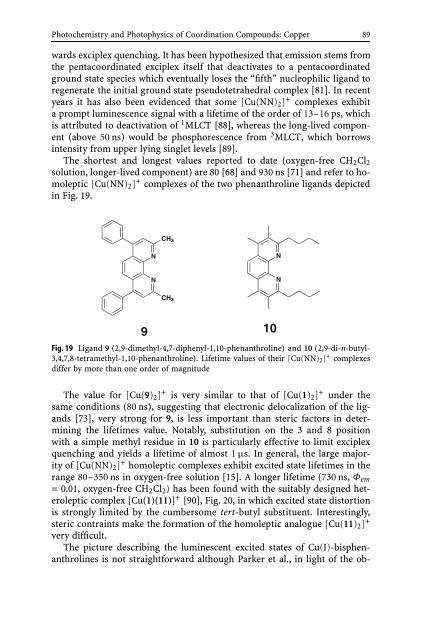
The shortest <strong>and</strong> longest values reported to date (oxygen-free CH2Cl2<br />
solution, longer-lived component) are 80 [68] <strong>and</strong> 930 ns [71] <strong>and</strong> refer to homoleptic<br />
[Cu(NN)2] + complexes <strong>of</strong> the two phenanthroline lig<strong>and</strong>s depicted<br />
in Fig. 19.<br />
Fig. 19 Lig<strong>and</strong> 9 (2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline) <strong>and</strong> 10 (2,9-di-n-butyl-<br />
3,4,7,8-tetramethyl-1,10-phenanthroline). Lifetime values <strong>of</strong> their [Cu(NN)2] + complexes<br />
differ by more than one order <strong>of</strong> magnitude<br />
The value for [Cu(9)2] + is very similar to that <strong>of</strong> [Cu(1)2] + under the<br />
same conditions (80 ns), suggesting that electronic delocalization <strong>of</strong> the lig<strong>and</strong>s<br />
[73], very strong for 9, is less important than steric factors in determining<br />
the lifetimes value. Notably, substitution on the 3 <strong>and</strong> 8 position<br />
with a simple methyl residue in 10 is particularly effective to limit exciplex<br />
quenching <strong>and</strong> yields a lifetime <strong>of</strong> almost 1 µs. In general, the large majority<br />
<strong>of</strong> [Cu(NN)2] + homoleptic complexes exhibit excited state lifetimes in the<br />
range 80–350 ns in oxygen-free solution [15]. A longer lifetime (730 ns, Φem<br />
= 0.01, oxygen-free CH2Cl2) has been found with the suitably designed heteroleptic<br />
complex [Cu(1)(11)] + [90], Fig. 20, in which excited state distortion<br />
is strongly limited by the cumbersome tert-butyl substituent. Interestingly,<br />
steric contraints make the formation <strong>of</strong> the homoleptic analogue [Cu(11)2] +<br />
very difficult.<br />
The picture describing the luminescent excited states <strong>of</strong> Cu(I)-bisphenanthrolinesisnotstraightforwardalthoughParkeretal.,inlight<strong>of</strong>theob-