Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Photochemistry</strong> <strong>and</strong> <strong>Photophysics</strong> <strong>of</strong> <strong>Coordination</strong> <strong>Compounds</strong>: Copper 85<br />
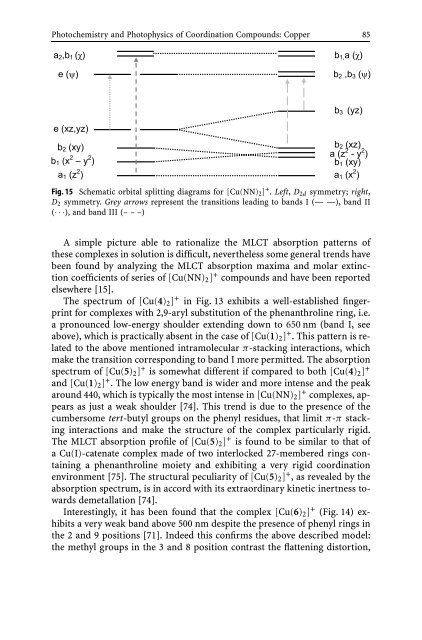
Fig. 15 Schematic orbital splitting diagrams for [Cu(NN)2] + . Left, D2d symmetry; right,<br />
D2 symmetry. Grey arrows represent the transitions leading to b<strong>and</strong>s I (— —), b<strong>and</strong> II<br />
(···), <strong>and</strong> b<strong>and</strong> III (– – –)<br />
A simple picture able to rationalize the MLCT absorption patterns <strong>of</strong><br />
these complexes in solution is difficult, nevertheless some general trends have<br />
been found by analyzing the MLCT absorption maxima <strong>and</strong> molar extinction<br />
coefficients <strong>of</strong> series <strong>of</strong> [Cu(NN)2] + compounds <strong>and</strong> have been reported<br />
elsewhere [15].<br />
The spectrum <strong>of</strong> [Cu(4)2] + in Fig. 13 exhibits a well-established fingerprint<br />
for complexes with 2,9-aryl substitution <strong>of</strong> the phenanthroline ring, i.e.<br />
a pronounced low-energy shoulder extending down to 650 nm (b<strong>and</strong> I, see<br />
above), which is practically absent in the case <strong>of</strong> [Cu(1)2] + .Thispatternisrelated<br />
to the above mentioned intramolecular π-stacking interactions, which<br />
make the transition corresponding to b<strong>and</strong> I more permitted. The absorption<br />
spectrum <strong>of</strong> [Cu(5)2] + is somewhat different if compared to both [Cu(4)2] +<br />
<strong>and</strong> [Cu(1)2] + . The low energy b<strong>and</strong> is wider <strong>and</strong> more intense <strong>and</strong> the peak<br />
around 440, which is typically the most intense in [Cu(NN)2] + complexes, appears<br />
as just a weak shoulder [74]. This trend is due to the presence <strong>of</strong> the<br />
cumbersome tert-butyl groups on the phenyl residues, that limit π-π stacking<br />
interactions <strong>and</strong> make the structure <strong>of</strong> the complex particularly rigid.<br />
The MLCT absorption pr<strong>of</strong>ile <strong>of</strong> [Cu(5)2] + is found to be similar to that <strong>of</strong><br />
aCu(I)-catenate complex made <strong>of</strong> two interlocked 27-membered rings containing<br />
a phenanthroline moiety <strong>and</strong> exhibiting a very rigid coordination<br />
environment [75]. The structural peculiarity <strong>of</strong> [Cu(5)2] + , as revealed by the<br />
absorption spectrum, is in accord with its extraordinary kinetic inertness towards<br />
demetallation [74].<br />
Interestingly, it has been found that the complex [Cu(6)2] + (Fig. 14) exhibits<br />
a very weak b<strong>and</strong> above 500 nm despite the presence <strong>of</strong> phenyl rings in<br />
the 2 <strong>and</strong> 9 positions [71]. Indeed this confirms the above described model:<br />
the methyl groups in the 3 <strong>and</strong> 8 position contrast the flattening distortion,