Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
168 S. Campagna et al.<br />
ground state, before excitation <strong>and</strong> any electron transfer process. The triad<br />
would then be better defined as a D/P–A system, <strong>and</strong> electron transfer between<br />
D <strong>and</strong> P + subunits in the intermediate state D/P + –A – can be largely<br />
faster than that reported for the D–P ∗ dyad, <strong>and</strong> even faster than charge<br />
recombination between C + <strong>and</strong> A – in the assembly, justifying the high efficiency<br />
<strong>of</strong> formation <strong>of</strong> the fully developed charge separation. Ground-state<br />
association <strong>of</strong> tethered aromatic lig<strong>and</strong>s (with flexible linkages) with Ru(II)<br />
chromophores was also known in a tetranuclear dendrimer [272, 273], further<br />
supporting these conclusions.<br />
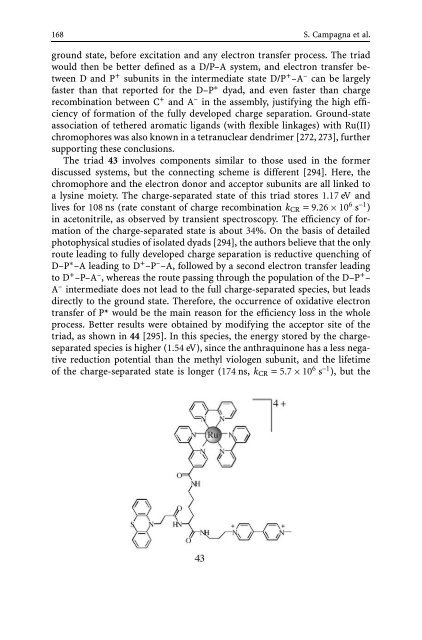
The triad 43 involves components similar to those used in the former<br />
discussed systems, but the connecting scheme is different [294]. Here, the<br />
chromophore <strong>and</strong> the electron donor <strong>and</strong> acceptor subunits are all linked to<br />
a lysine moiety. The charge-separated state <strong>of</strong> this triad stores 1.17 eV <strong>and</strong><br />
lives for 108 ns (rate constant <strong>of</strong> charge recombination kCR = 9.26 × 10 6 s –1 )<br />
in acetonitrile, as observed by transient spectroscopy. The efficiency <strong>of</strong> formation<br />
<strong>of</strong> the charge-separated state is about 34%. On the basis <strong>of</strong> detailed<br />
photophysical studies <strong>of</strong> isolated dyads [294], the authors believe that the only<br />
route leading to fully developed charge separation is reductive quenching <strong>of</strong><br />
D–P ∗ –A leading to D + –P – –A, followed by a second electron transfer leading<br />
to D + –P–A – , whereas the route passing through the population <strong>of</strong> the D–P + –<br />
A – intermediate does not lead to the full charge-separated species, but leads<br />
directly to the ground state. Therefore, the occurrence <strong>of</strong> oxidative electron<br />
transfer <strong>of</strong> P* would be the main reason for the efficiency loss in the whole<br />
process. Better results were obtained by modifying the acceptor site <strong>of</strong> the<br />
triad, as shown in 44 [295]. In this species, the energy stored by the chargeseparated<br />
species is higher (1.54 eV), since the anthraquinone has a less negative<br />
reduction potential than the methyl viologen subunit, <strong>and</strong> the lifetime<br />
<strong>of</strong> the charge-separated state is longer (174 ns, kCR = 5.7 × 10 6 s –1 ), but the