Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Photochemistry</strong> <strong>and</strong> <strong>Photophysics</strong> <strong>of</strong> <strong>Coordination</strong> <strong>Compounds</strong>: Rhodium 219<br />
properties <strong>of</strong> the very weak emission measured from room-temperature solutions<br />
<strong>of</strong> Rh(phen)3 3+ (b<strong>and</strong>shape, lifetimes) are consistent with a small<br />
amount <strong>of</strong> MC excited state being in thermal equilibrium with the lowest LC<br />
state [34]. In related 2,2 ′ -bipyridine complexes, changes in LC-MC energy gap<br />
<strong>and</strong> emission spectral pr<strong>of</strong>ile can be induced by methyl substitution in the<br />
3,3 ′ positions, as a consequence <strong>of</strong> changes in the degree <strong>of</strong> planarity <strong>of</strong> the<br />
lig<strong>and</strong> [37].<br />
The interplay <strong>of</strong> LC <strong>and</strong> MC triplets in the photophysics <strong>of</strong> this type <strong>of</strong><br />
complexes has been investigated in detail by studying the series <strong>of</strong> mixedlig<strong>and</strong><br />
complexes cis-Rh(phen)2XY n+ (X=Y=CN,n =1;X=Y=NH3, n =3;<br />
X=NH3, Y=Cl,n = 2), where the energy <strong>of</strong> the MC states is controlled by<br />
the lig<strong>and</strong> field strength <strong>of</strong> the X, Y ancillary lig<strong>and</strong>s [38]. While at room temperature<br />
all the complexes are very weakly emissive, at 77 K, the di-cyano <strong>and</strong><br />
di-amino complexes give the typical, structured LC emission, whereas the<br />
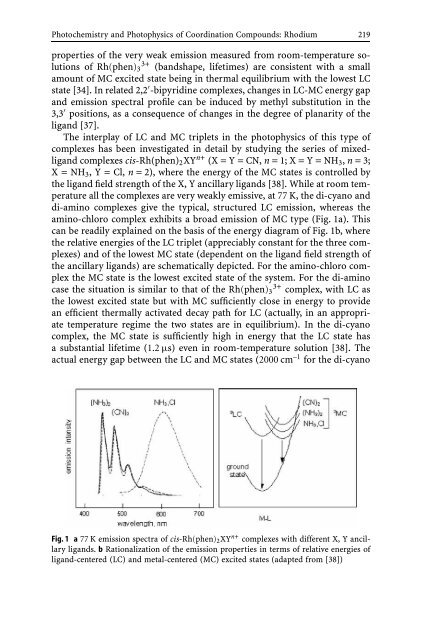
amino-chloro complex exhibits a broad emission <strong>of</strong> MC type (Fig. 1a). This<br />
can be readily explained on the basis <strong>of</strong> the energy diagram <strong>of</strong> Fig. 1b, where<br />
the relative energies <strong>of</strong> the LC triplet (appreciably constant for the three complexes)<br />
<strong>and</strong> <strong>of</strong> the lowest MC state (dependent on the lig<strong>and</strong> field strength <strong>of</strong><br />
the ancillary lig<strong>and</strong>s) are schematically depicted. For the amino-chloro complex<br />
the MC state is the lowest excited state <strong>of</strong> the system. For the di-amino<br />
case the situation is similar to that <strong>of</strong> the Rh(phen)3 3+ complex, with LC as<br />
the lowest excited state but with MC sufficiently close in energy to provide<br />
an efficient thermally activated decay path for LC (actually, in an appropriate<br />
temperature regime the two states are in equilibrium). In the di-cyano<br />
complex, the MC state is sufficiently high in energy that the LC state has<br />
a substantial lifetime (1.2 µs) even in room-temperature solution [38]. The<br />
actual energy gap between the LC <strong>and</strong> MC states (2000 cm –1 for the di-cyano<br />
Fig. 1 a 77 K emission spectra <strong>of</strong> cis-Rh(phen)2XY n+ complexes with different X, Y ancillary<br />
lig<strong>and</strong>s. b Rationalization <strong>of</strong> the emission properties in terms <strong>of</strong> relative energies <strong>of</strong><br />
lig<strong>and</strong>-centered (LC) <strong>and</strong> metal-centered (MC) excited states (adapted from [38])