Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
Photochemistry and Photophysics of Coordination Compounds
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Photochemistry</strong> <strong>and</strong> <strong>Photophysics</strong> <strong>of</strong> <strong>Coordination</strong> <strong>Compounds</strong>: Copper 109<br />
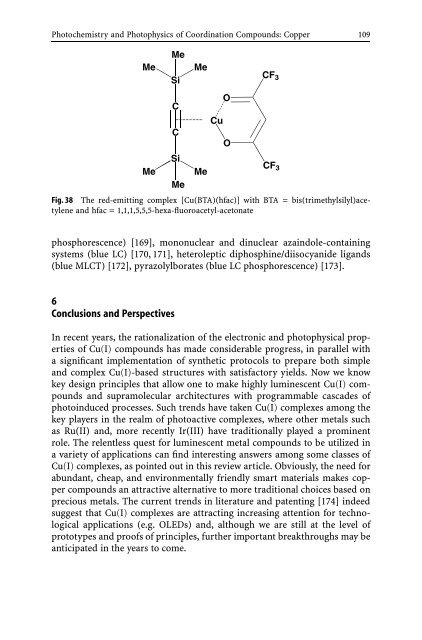
Fig. 38 The red-emitting complex [Cu(BTA)(hfac)] with BTA = bis(trimethylsilyl)acetylene<br />
<strong>and</strong> hfac = 1,1,1,5,5,5-hexa-fluoroacetyl-acetonate<br />
phosphorescence) [169], mononuclear <strong>and</strong> dinuclear azaindole-containing<br />
systems (blue LC) [170, 171], heteroleptic diphosphine/diisocyanide lig<strong>and</strong>s<br />
(blue MLCT) [172], pyrazolylborates (blue LC phosphorescence) [173].<br />
6<br />
Conclusions <strong>and</strong> Perspectives<br />
In recent years, the rationalization <strong>of</strong> the electronic <strong>and</strong> photophysical properties<br />
<strong>of</strong> Cu(I) compounds has made considerable progress, in parallel with<br />
a significant implementation <strong>of</strong> synthetic protocols to prepare both simple<br />
<strong>and</strong> complex Cu(I)-based structures with satisfactory yields. Now we know<br />
key design principles that allow one to make highly luminescent Cu(I) compounds<br />
<strong>and</strong> supramolecular architectures with programmable cascades <strong>of</strong><br />
photoinduced processes. Such trends have taken Cu(I) complexes among the<br />
key players in the realm <strong>of</strong> photoactive complexes, where other metals such<br />
as Ru(II) <strong>and</strong>, more recently Ir(III) have traditionally played a prominent<br />
role. The relentless quest for luminescent metal compounds to be utilized in<br />
a variety <strong>of</strong> applications can find interesting answers among some classes <strong>of</strong><br />
Cu(I) complexes, as pointed out in this review article. Obviously, the need for<br />
abundant, cheap, <strong>and</strong> environmentally friendly smart materials makes copper<br />
compounds an attractive alternative to more traditional choices based on<br />
precious metals. The current trends in literature <strong>and</strong> patenting [174] indeed<br />
suggest that Cu(I) complexes are attracting increasing attention for technological<br />
applications (e.g. OLEDs) <strong>and</strong>, although we are still at the level <strong>of</strong><br />
prototypes <strong>and</strong> pro<strong>of</strong>s <strong>of</strong> principles, further important breakthroughs may be<br />
anticipated in the years to come.