synthesis and in vitro pharmacology of a series of histamine h2 ...
synthesis and in vitro pharmacology of a series of histamine h2 ...
synthesis and in vitro pharmacology of a series of histamine h2 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapters<br />
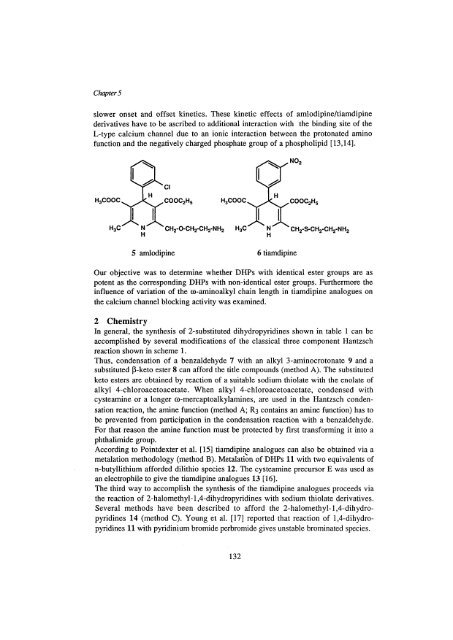
slower onset <strong>and</strong> <strong>of</strong>fset k<strong>in</strong>etics. These k<strong>in</strong>etic effects <strong>of</strong> amlodip<strong>in</strong>e/tiamdip<strong>in</strong>e<br />
derivatives have to be ascribed to additional <strong>in</strong>teraction with the b<strong>in</strong>d<strong>in</strong>g site <strong>of</strong> the<br />
L-type calcium channel due to an ionic <strong>in</strong>teraction between the protonated am<strong>in</strong>o<br />
function <strong>and</strong> the negatively charged phosphate group <strong>of</strong> a phospholipid [13,14].<br />
5 amlodip<strong>in</strong>e 6 tiamdip<strong>in</strong>e<br />
N0 2<br />
COOC 2H 5<br />
CH2 -<br />
S-CH2"CH2"NH2 Our objective was to determ<strong>in</strong>e whether DHPs with identical ester groups are as<br />
potent as the correspond<strong>in</strong>g DHPs with non-identical ester groups. Furthermore the<br />
<strong>in</strong>fluence <strong>of</strong> variation <strong>of</strong> the co-am<strong>in</strong>oalkyl cha<strong>in</strong> length <strong>in</strong> tiamdip<strong>in</strong>e analogues on<br />
the calcium channel block<strong>in</strong>g activity was exam<strong>in</strong>ed.<br />
2 Chemistry<br />
In general, the <strong>synthesis</strong> <strong>of</strong> 2-substituted dihydropyrid<strong>in</strong>es shown <strong>in</strong> table 1 can be<br />
accomplished by several modifications <strong>of</strong> the classical three component Hantzsch<br />
reaction shown <strong>in</strong> scheme 1.<br />
Thus, condensation <strong>of</strong> a benzaldehyde 7 with an alkyl 3-am<strong>in</strong>ocrotonate 9 <strong>and</strong> a<br />
substituted p-keto ester 8 can afford the title compounds (method A). The substituted<br />
keto esters are obta<strong>in</strong>ed by reaction <strong>of</strong> a suitable sodium thiolate with the enolate <strong>of</strong><br />
alkyl 4-chloroacetoacetate. When alkyl 4-chloroacetoacetate, condensed with<br />
cysteam<strong>in</strong>e or a longer co-mercaptoalkylam<strong>in</strong>es, are used <strong>in</strong> the Hantzsch condensation<br />
reaction, the am<strong>in</strong>e function (method A; R3 conta<strong>in</strong>s an am<strong>in</strong>e function) has to<br />
be prevented from participation <strong>in</strong> the condensation reaction with a benzaldehyde.<br />
For that reason the am<strong>in</strong>e function must be protected by first transform<strong>in</strong>g it <strong>in</strong>to a<br />
phthalimide group.<br />
Accord<strong>in</strong>g to Po<strong>in</strong>tdexter et al. [15] tiamdip<strong>in</strong>e analogues can also be obta<strong>in</strong>ed via a<br />
metalation methodology (method B). Metalation <strong>of</strong> DHPs 11 with two equivalents <strong>of</strong><br />
n-butyllithium afforded dilithio species 12. The cysteam<strong>in</strong>e precursor E was used as<br />
an electrophile to give the tiamdip<strong>in</strong>e analogues 13 [16].<br />
The third way to accomplish the <strong>synthesis</strong> <strong>of</strong> the tiamdip<strong>in</strong>e analogues proceeds via<br />
the reaction <strong>of</strong> 2-halomethyl-l,4-dihydropyrid<strong>in</strong>es with sodium thiolate derivatives.<br />
Several methods have been described to afford the 2-halomethyl-l,4-dihydropyrid<strong>in</strong>es<br />
14 (method C). Young et al. [17] reported that reaction <strong>of</strong> 1,4-dihydropyrid<strong>in</strong>es<br />
11 with pyrid<strong>in</strong>ium bromide perbromide gives unstable brom<strong>in</strong>ated species.<br />
132