synthesis and in vitro pharmacology of a series of histamine h2 ...
synthesis and in vitro pharmacology of a series of histamine h2 ...
synthesis and in vitro pharmacology of a series of histamine h2 ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 6<br />
Synthesis <strong>and</strong> <strong>in</strong> <strong>vitro</strong> <strong>pharmacology</strong> <strong>of</strong> a <strong>series</strong> <strong>of</strong> new 1,4dihydropyrid<strong>in</strong>es.<br />
2.<br />
Diethyl 4-[2-(co-am<strong>in</strong>oalkoxy)phenyl]-2,6-dimethyl-l,4-dihydropyrid<strong>in</strong>e-3,5-dicarboxylates<br />
<strong>and</strong> their correspond<strong>in</strong>g<br />
isothioureas as tools for determ<strong>in</strong><strong>in</strong>g structure-activity<br />
relationships 1<br />
Chapter 6<br />
1 Introduction<br />
The <strong>in</strong>troduction <strong>of</strong> 4-aryl-l,4-dihydropyrid<strong>in</strong>es (DHPs) with highly potent Ca 2+<br />
-<br />
channel block<strong>in</strong>g activity led to a new direction <strong>in</strong> cardiovascular therapy. The Ca 2+<br />
-<br />
channel blockers are now well established <strong>in</strong> the treatment <strong>of</strong> ang<strong>in</strong>a pectoris,<br />
hypertension, certa<strong>in</strong> cardiac arrhythmias <strong>and</strong> peripheral vascular disorders [1-5]. The<br />
1,4-dihydropyrid<strong>in</strong>e Ca 2+<br />
2 +<br />
-channel blockers <strong>in</strong>hibit the <strong>in</strong>flux <strong>of</strong> extracellular Ca via<br />
L-type potential-dependent calcium channels <strong>and</strong> reduce vascular resistance.<br />
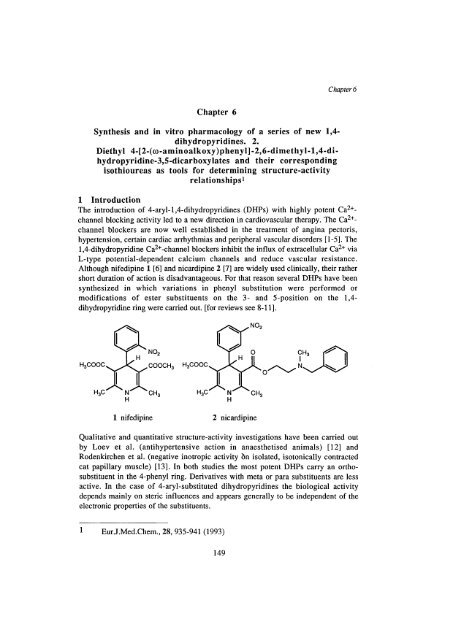
Although nifedip<strong>in</strong>e 1 [6] <strong>and</strong> nicardip<strong>in</strong>e 2 [7] are widely used cl<strong>in</strong>ically, their rather<br />
short duration <strong>of</strong> action is disadvantageous. For that reason several DHPs have been<br />
synthesized <strong>in</strong> which variations <strong>in</strong> phenyl substitution were performed or<br />
modifications <strong>of</strong> ester substituents on the 3- <strong>and</strong> 5-position on the 1,4dihydropyrid<strong>in</strong>e<br />
r<strong>in</strong>g were carried out. [for reviews see 8-11].<br />
H H<br />
1 nifedip<strong>in</strong>e 2 nicardip<strong>in</strong>e<br />
Qualitative <strong>and</strong> quantitative structure-activity <strong>in</strong>vestigations have been carried out<br />
by Loev et al. (antihypertensive action <strong>in</strong> anaesthetised animals) [12] <strong>and</strong><br />
Rodenkirchen et al. (negative <strong>in</strong>otropic activity 5n isolated, isotonically contracted<br />
cat papillary muscle) [13]. In both studies the most potent DHPs carry an orthosubstituent<br />
<strong>in</strong> the 4-phenyl r<strong>in</strong>g. Derivatives with meta or para substituents are less<br />
active. In the case <strong>of</strong> 4-aryl-substituted dihydropyrid<strong>in</strong>es the biological activity<br />
depends ma<strong>in</strong>ly on steric <strong>in</strong>fluences <strong>and</strong> appears generally to be <strong>in</strong>dependent <strong>of</strong> the<br />
electronic properties <strong>of</strong> the substituents.<br />
EurJ.Med.Chem., 28, 935-941 (1993)<br />
149