synthesis and in vitro pharmacology of a series of histamine h2 ...
synthesis and in vitro pharmacology of a series of histamine h2 ...
synthesis and in vitro pharmacology of a series of histamine h2 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 2<br />
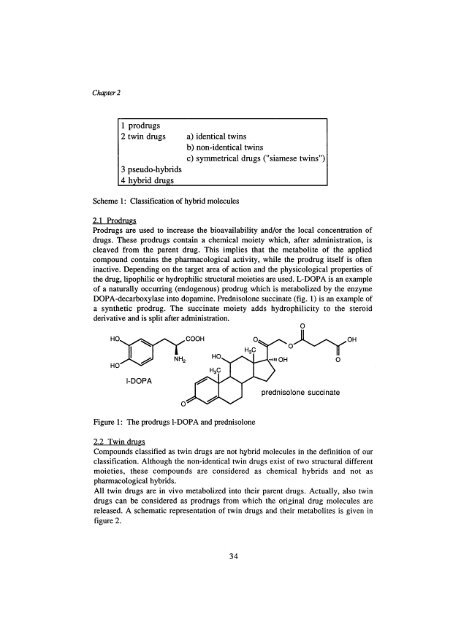
1 prodrugs<br />
2 tw<strong>in</strong> drugs a) identical tw<strong>in</strong>s<br />
3 pseudo-hybrids<br />
4 hybrid drugs<br />
b) non-identical tw<strong>in</strong>s<br />
Scheme 1: Classification <strong>of</strong> hybrid molecules<br />
2.1 Prodrugs<br />
c) symmetrical drugs ("siamese tw<strong>in</strong>s")<br />
Prodrugs are used to <strong>in</strong>crease the bioavailability <strong>and</strong>/or the local concentration <strong>of</strong><br />
drugs. These prodrugs conta<strong>in</strong> a chemical moiety which, after adm<strong>in</strong>istration, is<br />
cleaved from the parent drug. This implies that the metabolite <strong>of</strong> the applied<br />
compound conta<strong>in</strong>s the pharmacological activity, while the prodrug itself is <strong>of</strong>ten<br />
<strong>in</strong>active. Depend<strong>in</strong>g on the target area <strong>of</strong> action <strong>and</strong> the physicological properties <strong>of</strong><br />
the drug, lipophilic or hydrophilic structural moieties are used. L-DOPA is an example<br />
<strong>of</strong> a naturally occurr<strong>in</strong>g (endogenous) prodrug which is metabolized by the enzyme<br />
DOPA-decarboxylase <strong>in</strong>to dopam<strong>in</strong>e. Prednisolone succ<strong>in</strong>ate (fig. 1) is an example <strong>of</strong><br />
a synthetic prodrug. The succ<strong>in</strong>ate moiety adds hydrophilicity to the steroid<br />
derivative <strong>and</strong> is split after adm<strong>in</strong>istration.<br />
O<br />
Figure 1: The prodrugs 1-DOPA <strong>and</strong> prednisolone<br />
2.2 Tw<strong>in</strong> drugs<br />
O<br />
Compounds classified as tw<strong>in</strong> drugs are not hybrid molecules <strong>in</strong> the def<strong>in</strong>ition <strong>of</strong> our<br />
classification. Although the non-identical tw<strong>in</strong> drugs exist <strong>of</strong> two structural different<br />
moieties, these compounds are considered as chemical hybrids <strong>and</strong> not as<br />
pharmacological hybrids.<br />
All tw<strong>in</strong> drugs are <strong>in</strong> vivo metabolized <strong>in</strong>to their parent drugs. Actually, also tw<strong>in</strong><br />
drugs can be considered as prodrugs from which the orig<strong>in</strong>al drug molecules are<br />
released. A schematic representation <strong>of</strong> tw<strong>in</strong> drugs <strong>and</strong> their metabolites is given <strong>in</strong><br />
figure 2.<br />
34