OptiSeal
OptiSeal
OptiSeal
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>OptiSeal</strong> Valved<br />
PTFE Peelable Introducer<br />
Instructions for Use<br />
Device Description<br />
The <strong>OptiSeal</strong> Valved PTFE Peelable Introducer<br />
is designed to reduce blood loss and air intake by<br />
providing hemostatic sealing at venous pressures.<br />
The device is offered in two configurations, one<br />
without a sideport and one with a sideport attached<br />
to a segment of extension tubing terminating in a<br />
3-way stopcock.<br />
Intended Use<br />
The <strong>OptiSeal</strong> Valved PTFE Peelable Introducer<br />
is intended for use in the percutaneous insertion of<br />
pacing leads or catheters in the venous system.<br />
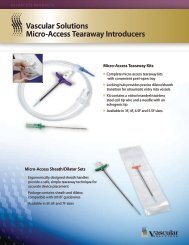
Sterile Kit Contents<br />
• 1 Introducer (with or without sideport)<br />
• 1 Guidewire (.035”/.89mm)<br />
• 1 Needle (18 gauge)<br />
• 1 Syringe (12cc)<br />
Contraindications<br />
Use of this product is contraindicated if the patient<br />
has a known or suspected obstruction in the<br />
intended access vein. There is increased risk of<br />
pneumothorax for the patient who has severe<br />
chronic lung disease. Poor healing may result in<br />
the patient who has had irradiation to the anterior<br />
chest.<br />
Precautions<br />
• Store in dry, dark, cool place.<br />
• Do not use if package is opened or damaged.<br />
• Inspect all components prior to use.<br />
• Product was sterilized using Ethylene Oxide.<br />
• The introducer shall be used only with<br />
components contained in this kit and is<br />
intended for use with leads or catheters with<br />
the same or smaller diameter than the inner<br />
diameter stated on the label.<br />
Cautions<br />
• Product may only be used by medically<br />
skilled personnel thoroughly trained in this<br />
procedure.<br />
• Product not intended for arterial use as a<br />
hemostatic device. Insertion into an artery<br />
may cause excessive bleeding and/or other<br />
complications.<br />
• Only use the sideport to inject or aspirate<br />
through the sheath.<br />
2<br />
• The product is intended for use with leads<br />
and catheters with a smaller diameter than<br />
the inner diameter of the sheath.<br />
• Dilators, leads, and catheters should be<br />
removed slowly from the product. Rapid<br />
removal may damage the valve members<br />
resulting in blood flow through the valve.<br />
• Do not attempt to use a guidewire over the<br />
maximum diameter of .038 inches or<br />
.965 millimeters.<br />
• Do not over torque the dilator nut.<br />
• If resistance is met when advancing or<br />
withdrawing the guidewire or the introducer,<br />
determine the cause by fluoroscopy and<br />
correct before continuing with the procedure.<br />
• Do not use forceps to break the handle<br />
and/or to peel the sheath.<br />
• Apply symmetrical force while peeling the<br />
sheath.<br />
• Individual patient anatomy and physician<br />
technique may require procedural variations.<br />
• Federal (USA) law restricts this device to sale<br />
by or on the order of a physician.<br />
Warnings<br />
• Do not alter this product in any way.<br />
• Do not use alcohol, acetone or solutions<br />
containing these agents. These solutions<br />
may affect the properties of the plastic<br />
components resulting in degradation of the<br />
product and compromised performance.<br />
• Ensure all air is removed from the product<br />
prior to infusing through the sideport.<br />
• Do not aspirate through the sideport while<br />
the guidewire is positioned in the valve of<br />
the sheath.<br />
• Aspiration with the guidewire through the<br />
valve may cause an air embolism.<br />
• Do not withdraw guidewire through metal<br />
needles; guidewire may shear or unravel.<br />
• For single use only. DO NOT REUSE,<br />
DO NOT RESTERILIZE.<br />
• Reuse of single-use devices creates a<br />
potential risk of patient or user infections.<br />
• Contamination of the device may lead<br />
to injury, illness or death of the patient.<br />
Cleaning, disinfection and sterilization may<br />
compromise essential material and design<br />
characteristics leading to device failure.<br />
Potential Complications<br />
The potential complications related to the use<br />
of the product include, but are not limited to the<br />
following:<br />
• Air Embolism<br />
• Artery puncture<br />
• Pneumothorax<br />
• Hemothorax<br />
• Hematoma formation<br />
• Brachial plexus injury<br />
• Vein thrombosis