Diploma thesis in Physics submitted by Florian Freundt born in ...
Diploma thesis in Physics submitted by Florian Freundt born in ...
Diploma thesis in Physics submitted by Florian Freundt born in ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.1. Noble gas temperatures 2 Theory<br />
� � � � � � � � � � � �� � � �<br />
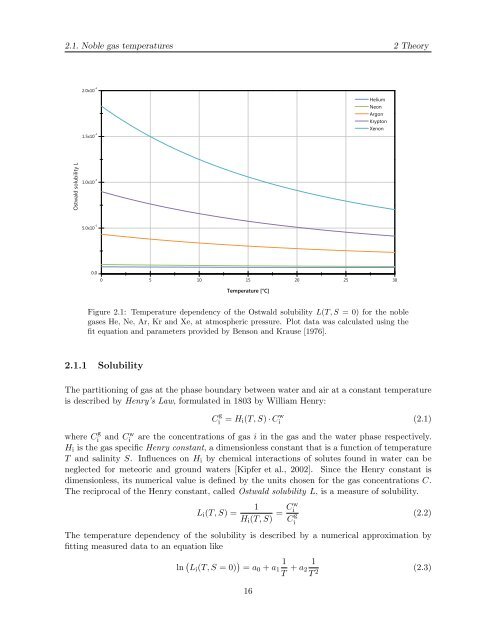
Figure 2.1: Temperature dependency of the Ostwald solubility L(T, S = 0) for the noble<br />
gases He, Ne, Ar, Kr and Xe, at atmospheric pressure. Plot data was calculated us<strong>in</strong>g the<br />
fit equation and parameters provided <strong>by</strong> Benson and Krause [1976].<br />
2.1.1 Solubility<br />
The partition<strong>in</strong>g of gas at the phase boundary between water and air at a constant temperature<br />
is described <strong>by</strong> Henry’s Law, formulated <strong>in</strong> 1803 <strong>by</strong> William Henry:<br />
C g<br />
i = Hi(T, S) · C w i (2.1)<br />
where C g<br />
i and Cw i are the concentrations of gas i <strong>in</strong> the gas and the water phase respectively.<br />
Hi is the gas specific Henry constant, a dimensionless constant that is a function of temperature<br />
T and sal<strong>in</strong>ity S. Influences on Hi <strong>by</strong> chemical <strong>in</strong>teractions of solutes found <strong>in</strong> water can be<br />
neglected for meteoric and ground waters [Kipfer et al., 2002]. S<strong>in</strong>ce the Henry constant is<br />
dimensionless, its numerical value is def<strong>in</strong>ed <strong>by</strong> the units chosen for the gas concentrations C.<br />
The reciprocal of the Henry constant, called Ostwald solubility L, is a measure of solubility.<br />
Li(T, S) =<br />
1<br />
Hi(T, S) = Cw i<br />
C g<br />
i<br />
The temperature dependency of the solubility is described <strong>by</strong> a numerical approximation <strong>by</strong><br />
fitt<strong>in</strong>g measured data to an equation like<br />
ln � Li(T, S = 0) � 1 1<br />
= a0 + a1 + a2<br />
T<br />
16<br />
T 2<br />
(2.2)<br />
(2.3)