Diploma thesis in Physics submitted by Florian Freundt born in ...
Diploma thesis in Physics submitted by Florian Freundt born in ...
Diploma thesis in Physics submitted by Florian Freundt born in ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2 Theory 2.3. Soil atmosphere composition<br />
Respiration rate K [g CO2 m-2 d-1]<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0<br />
Jan<br />
Mar Apr<br />
Feb<br />
Dec<br />
Nov<br />
5 10<br />
K0 = 1.2<br />
May<br />
Oct<br />
Aug<br />
Jun<br />
K0 = 0.9<br />
average soil temperature at 10 cm depth [°C]<br />
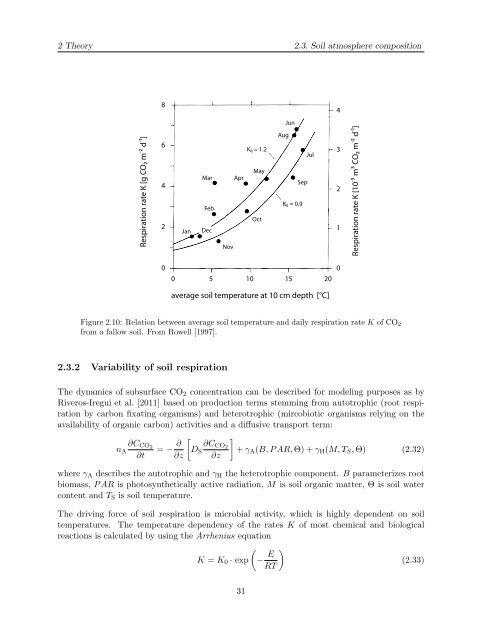
Figure 2.10: Relation between average soil temperature and daily respiration rate K of CO2<br />
from a fallow soil. From Rowell [1997].<br />
2.3.2 Variability of soil respiration<br />
The dynamics of subsurface CO2 concentration can be described for model<strong>in</strong>g purposes as <strong>by</strong><br />
Riveros-Iregui et al. [2011] based on production terms stemm<strong>in</strong>g from autotrophic (root respiration<br />
<strong>by</strong> carbon fixat<strong>in</strong>g organisms) and heterotrophic (mircobiotic organisms rely<strong>in</strong>g on the<br />
availability of organic carbon) activities and a diffusive transport term:<br />
∂CCO2<br />
nA<br />
∂t<br />
= − ∂<br />
∂z<br />
�<br />
DS<br />
∂CCO2<br />
∂z<br />
15<br />
Sep<br />
Jul<br />
20<br />
4<br />
3<br />
2<br />
1<br />
0<br />
Respiration rate K [10-3 m3 CO2 m-2 d-1]<br />
�<br />
+ γA(B, P AR, Θ) + γH(M, TS, Θ) (2.32)<br />
where γA describes the autotrophic and γH the heterotrophic component. B parameterizes root<br />
biomass, P AR is photosynthetically active radiation, M is soil organic matter, Θ is soil water<br />
content and TS is soil temperature.<br />
The driv<strong>in</strong>g force of soil respiration is microbial activity, which is highly dependent on soil<br />
temperatures. The temperature dependency of the rates K of most chemical and biological<br />
reactions is calculated <strong>by</strong> us<strong>in</strong>g the Arrhenius equation<br />
�<br />
K = K0 · exp − E<br />
�<br />
(2.33)<br />
RT<br />
31